Mutagenicity of dibenz[a,c]anthracene and its derivatives in Salmonella typhimurium TA100.
S Kumar, P L Kole, H C Sikka
文献索引:Mutat. Res. 242(4) , 337-43, (1990)
全文:HTML全文
摘要
The mutagenic activities of dibenz[a,c]anthracene (DB[a,c]A), and its 11 derivatives, including 3 diols, 6 phenols and 2 oxepines, were studied in the TA100 strain of Salmonella typhimurium at doses varying from 0 to 20 micrograms/plate in the presence of a rat-liver S9 (9000 x g) preparation. Among the diols of DB[a,c]A tested DB[a,c]A-10,11-diol was the most mutagenic compound. However, it was consistently less mutagenic than the parent hydrocarbon. Oxepine-1 and oxepine-2 which are believed to be the photoisomerized products of DB[a,c]A-1,2 oxide and DB[a,c]A-3,4-oxide, respectively, were also less mutagenic than DB[a,c]A. In contrast to these results, 4-hydroxyDB[a,c]A was almost twice as active as DB[a,c]A, and 2-hydroxy- and 3-hydroxyDB[a,c]A were even more (4-6-fold) mutagenic than DB[a,c]A. The remaining phenols were relatively inactive or weakly active in this mutagenicity assay. These results provide initial evidence that the bay-region theory may not be applicable to the mutagenesis of DB[a,c]A, and that the angular ring substituted phenols of DB[a,c]A may be involved in the metabolic activation of this highly mutagenic hydrocarbon.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
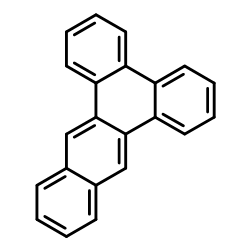 |
1,2,3,4-二苯并蒽
CAS:215-58-7 |
C22H14 |
|
Determination of polycyclic aromatic hydrocarbons in fat pro...
1980-07-01 [Z. Lebensm. Unters. Forsch. 171(1) , 9-13, (1980)] |
|
Infrared matrix-assisted laser desorption/ionization of poly...
2001-01-01 [Rapid Commun. Mass Spectrom. 15(16) , 1448-52, (2001)] |
|
Microsomal metabolism of dibenz[a,c]anthracene, dibenz[a,h]a...
1991-01-01 [Chem. Biol. Interact. 80(3) , 261-79, (1991)] |
|
Comparison of the in vitro metabolisms and mutagenicities of...
1989-03-01 [Carcinogenesis 10(3) , 461-9, (1989)] |
|
The metabolic activation of some polycyclic hydrocarbons: th...
1981-01-01 [Adv. Exp. Med. Biol. 136 Pt A , 487-500, (1981)] |