Utility of the ammonia-free Birch reduction of electron-deficient pyrroles: total synthesis of the 20s proteasome inhibitor, clasto-lactacystin beta-lactone.
Timothy J Donohoe, Herman O Sintim, Leena Sisangia, Karl W Ace, Paul M Guyo, Andrew Cowley, John D Harling
文献索引:Chemistry 11(14) , 4227-38, (2005)
全文:HTML全文
摘要
A new synthesis of the 20S proteasome inhibitor clasto-lactacystin beta-lactone is described. Our route to this important natural product involves the partial reduction of an electron deficient pyrrole as a key step. By judicious choice of enolate counterion, we were able to exert complete control over the stereoselectivity of the reduction/aldol reaction. Early attempts to complete the synthesis by using a C-4 methyl substituted pyrrole are described in full, together with our attempts to promote regioselective elimination of a tertiary alcohol. The lessons learnt from this first approach led us to develop another, and ultimately successful, route that introduced the C-4 methyl group at a late stage in the synthesis. Our successful route is then described and this contains several highly stereoselective steps including a cis-dihydroxylation and an enolate methylation. The final synthesis proceeds in just 13 steps and in 15 % overall yield making it an extremely efficient route to this valuable compound.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
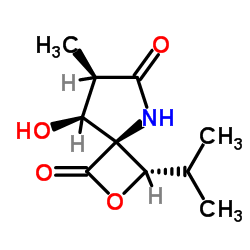 |
Omuralide
CAS:154226-60-5 |
C10H15NO4 |
|
Proteasome inhibitor differentially regulates expression of ...
2009-06-01 [Virus Res. 142(1-2) , 68-77, (2009)] |
|
Turnover of StAR protein: roles for the proteasome and mitoc...
2007-02-01 [Mol. Cell. Endocrinol. 265-266 , 51-8, (2007)] |
|
Targeting of a chlamydial protease impedes intracellular bac...
2011-09-01 [PLoS Pathog. 7(9) , e1002283, (2011)] |
|
Activity dependent protein degradation is critical for the f...
2011-01-01 [PLoS ONE 6(9) , e24349, (2011)] |
|
Proteasome inhibition triggers activity-dependent increase i...
2006-11-01 [J. Neurosci. 26(44) , 11333-41, (2006)] |