NMR spectroscopic analysis of new spiro-piperidylrifamycins.
Eduardo Rubio, Isabel Merino, Ana-Belén García, María-Paz Cabal, Cristina Ribas, Miguel Bayod-Jasanada
文献索引:Magn. Reson. Chem. 43(4) , 269-82, (2005)
全文:HTML全文
摘要
New spiro-piperidylrifamycin derivatives are presented. These compounds were synthesized from the reaction of 3-amino-4-iminorifamycin S and enantiomerically pure 4-piperidones, which generate diasteroisomeric rifabutin analogues with a new stereocentre at the spiranic carbon. The (1)H and (13)C NMR spectra of these new compounds, and also the configuration of the new stereogenic centres, were assigned using 2D NMR spectroscopic techniques. A preliminary study of the (1)H and (13)C NMR spectra of the starting compounds rifamycin S, 3-amino-4-iminorifamycin S and the related rifabutin was also carried out and as a result, their previously published (13)C NMR data were corrected.Copyright (c) 2005 John Wiley & Sons, Ltd
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
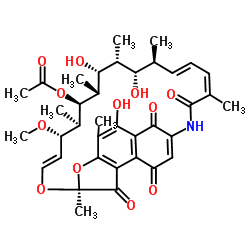 |
利福霉素S
CAS:13553-79-2 |
C37H45NO12 |
|
Polyketide construction via hydrohydroxyalkylation and relat...
2014-04-01 [Nat. Prod. Rep. 31(4) , 504-13, (2014)] |
|
Synthesis and structure-activity relationships of novel subs...
2011-10-15 [Bioorg. Med. Chem. Lett. 21 , 6094-9, (2011)] |
|
Direct generation of acyclic polypropionate stereopolyads vi...
2011-08-17 [J. Am. Chem. Soc. 133(32) , 12795-800, (2011)] |
|
Detoxification of toxins by bacillithiol in Staphylococcus a...
2012-04-01 [Microbiology 158(Pt 4) , 1117-26, (2012)] |
|
Solid state cultivation of Curvularia lunata for transformat...
2002-08-01 [Indian J. Exp. Biol. 40(8) , 930-3, (2002)] |