NAD+-specific D-arabinose dehydrogenase and its contribution to erythroascorbic acid production in Saccharomyces cerevisiae.
Katsumi Amako, Kazuyo Fujita, Taka-aki Shimohata, Etsuko Hasegawa, Ritsuko Kishimoto, Kiyoshi Goda
文献索引:FEBS Lett. 580(27) , 6428-34, (2006)
全文:HTML全文
摘要
Erythroascorbic acid (eAsA) is a five-carbon analog of ascorbic acid, and it is synthesized from D-arabinose by D-arabinose dehydrogenase (ARA) and D-arabinono-gamma-lactone oxidase. We found an NAD+-specific ARA activity which is operative under submillimolar level of d-arabinose in the extracts of Saccharomyces cerevisiae. The hypothetical protein encoded by YMR041c showed a significant homology to a l-galactose dehydrogenase which plays in plant ascorbic acid biosynthesis, and we named it as Ara2p. Recombinant Ara2p showed NAD+-specific ARA activity with Km=0.78 mM to d-arabinose, which is 200-fold lower than that for the conventional NADP+-specific ARA, Ara1p. Gene disruptant of ARA2 lost entire NAD+-specific ARA activity and the conspicuous increase in intracellular eAsA by exogenous d-arabinose feeding, while the double knockout mutant of ARA1 and ARA2 still retained measurable amount of eAsA. It demonstrates that Ara2p, not Ara1p, mainly contributes to the production of eAsA from d-arabinose in S. cerevisiae.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
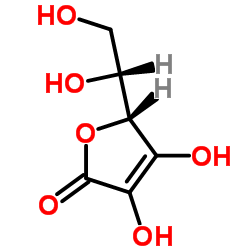 |
D-异抗坏血酸
CAS:89-65-6 |
C6H8O6 |
|
Effect of meat ingredients (sodium nitrite and erythorbate) ...
2013-09-01 [Food Microbiol. 35(2) , 108-15, (2013)] |
|
The effect of antibrowning agents on inhibition of potato br...
2012-11-01 [J. Food Sci. 77(11) , C1234-40, (2012)] |
|
Study on mitigation of acrylamide formation in cookies by 5 ...
2012-11-01 [J. Food Sci. 77(11) , C1144-9, (2012)] |
|
Determination of isoascorbic acid in fish tissue by hydrophi...
2010-07-01 [Anal. Bioanal. Chem 397(6) , 2199-210, (2010)] |
|
Mechanism of L-ascorbic acid uptake by rabbit corneal epithe...
2006-06-01 [Curr. Eye Res. 31(6) , 481-9, (2006)] |