Thiol metabolomics of endothelial cells using capillary liquid chromatography mass spectrometry with isotope coded affinity tags.
Wei Yuan, James L Edwards
文献索引:J. Chromatogr. A. 1218(18) , 2561-8, (2011)
全文:HTML全文
摘要
Thiol and disulfide levels are critical to maintaining the redox potential of a cell. Perturbations of these levels are important in disease pathogenesis. To improve endogenous mammalian metabolome quantitation, thiol specific tagging, extraction and relative quantitation were undertaken. Reduced and oxidized thiol (disulfide) metabolites from endothelial cells were tagged and extracted using cleavable isotope coded affinity tags (cICAT). Extracted cICAT labeled thiols were analyzed using capillary reverse phase liquid chromatography coupled to mass spectrometry (capLC-MS) with positive mode electrospray ionization. Reactions between thiol metabolite standards and the reactive group of cICAT indicate completion by 8h at pH 9 with no apparent disulfide formation. cICAT labeled reduced thiols from endothelial cells showed 1-5% RSD using ratiometric quantitation of isotopes and 6-17% RSD based on signal intensity alone. Sample injection was optimized to 16 pmol. Using high mass accuracy MS, 75 putative thiol metabolites were detected in all experimental samples. Treatment of endothelial cells with 2,3-dimethoxy-5-methyl-1,4-benzoquinone (BQ) shows decreased levels in 28 putative reduced thiols and increased levels of 27 putative disulfides. Treatment of endothelial cells with 30 mM glucose resulted in 22 putative reduced thiols with decreased levels and 7 putative disulfides with increased concentration. Thiols were identified based on accurate mass within 3 ppm and analysis of fragmentation patterns. Using higher collision induced dissociation (HCD), shared product ions between different thiols led to the analysis of thiols from the cysteine-glutathione (Cys-GSH) pathway. Specific reduced thiols and disulfides in this pathway revealed changes different from the overall trends of thiols/disulfides. This suggests varying regulation of the Cys-GSH pathway distinct from other thiol-containing pathways and dependence on the type of environmental stimulus. These results indicate the utility of analyzing reduced thiols and disulfides in eukaryotic samples.Copyright © 2011 Elsevier B.V. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
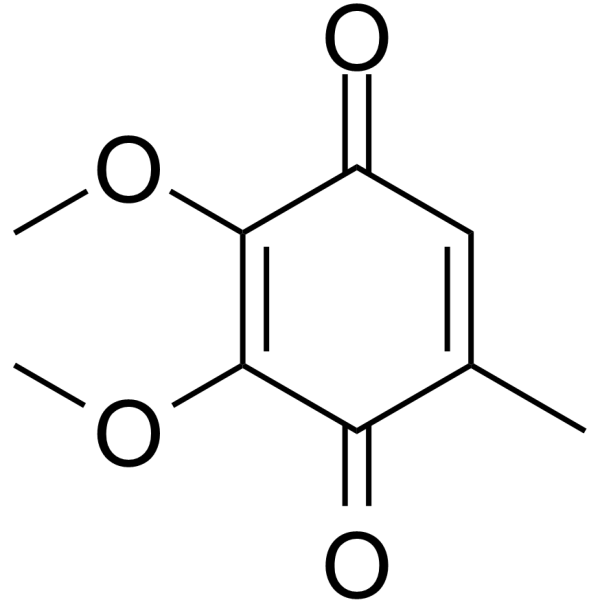 |
2,3-二甲氧基-5-甲基-1,4-苯醌
CAS:605-94-7 |
C9H10O4 |
|
Cofactor Specificity of the Bifunctional Alcohol and Aldehyd...
2015-08-01 [J. Bacteriol. 197 , 2610-9, (2015)] |
|
Prerequisites for ubiquinone analogs to prevent mitochondria...
2012-02-01 [J. Bioenerg. Biomembr. 44(1) , 207-12, (2012)] |
|
Ubiquinone analogs: a mitochondrial permeability transition ...
2010-01-01 [PLoS ONE 5 , e11792, (2010)] |
|
Immunochemical identification of coenzyme Q0-dihydrolipoamid...
2004-06-25 [J. Biol. Chem. 279 , 27278-27285, (2004)] |
|
Photolabile ubiquinone analogues for identification and char...
2010-01-01 [Bioorg. Med. Chem. 18 , 3457-66, (2010)] |