Synthesis and biological evaluation of guanidino compounds endowed with subnanomolar affinity as competitive inhibitors of maize polyamine oxidase.
Fabrizio Manetti, Alessandra Cona, Lucilla Angeli, Claudia Mugnaini, Francesco Raffi, Caterina Capone, Elena Dreassi, Alessandra Tania Zizzari, Alessandra Tisi, Rodolfo Federico, Maurizio Botta
文献索引:J. Med. Chem. 52 , 4774-85, (2009)
全文:HTML全文
摘要
Previous studies on agmatine and its derivatives suggested that the presence of hydrophobic groups on the guanidine moiety was a crucial key for inhibitory activity of maize polyamine oxidase. Accordingly, new lipophilic agmatine and iminoctadine derivatives were synthesized and tested for their ability to inhibit this enzyme. Several compounds showed an affinity in the nanomolar range, while a cyclopropylmethyl derivative of iminoctadine was found to be the most potent inhibitor of maize polyamine oxidase reported so far (Ki = 0.08 nM).
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
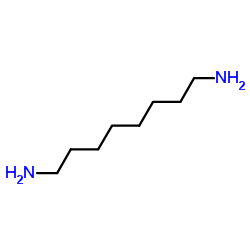 |
1,8-辛二胺
CAS:373-44-4 |
C8H20N2 |
|
Paralcaligenes ginsengisoli sp. nov., isolated from ginseng ...
2015-09-01 [Antonie van Leeuwenhoek 108 , 619-26, (2015)] |
|
Enhanced transcellular penetration and drug delivery by cros...
2015-07-01 [Biomater. Sci. 3 , 1085-95, (2015)] |
|
An easy-to-use excimer fluorescence derivatization reagent, ...
2015-06-23 [Anal. Chim. Acta 880 , 145-51, (2015)] |
|
New Trend for Acceleration Solid Phase Extraction Process Ba...
2015-01-01 [Anal. Sci. 31 , 1047-54, (2015)] |
|
Nanobioconjugates of Candida antarctica lipase B and single-...
2016-01-01 [Bioresour. Technol. 200 , 853-60, (2015)] |