Preparation and preliminary biodistribution of "no carrier added" fluorine1-8 fluoroethanol.
T J Tewson, M J Welch
文献索引:J. Nucl. Med. 21(6) , 559-64, (1980)
全文:HTML全文
摘要
No-carrier-added [18F] fluoroethanol has been prepared by two routes. The first involves fluoride-ion displacement on alpha-p-toluene sulfonyl ethyl glycolate followed by reduction of the alpha-fluoroethyl acetate. The second involves a ring-opening reaction on glycol sulfite to give an alpha-fluorosulfinic acid derivative that is hydrolyzed to fluoroethanol. The specific activity was measured as 10(5) Ci/millimole, and the stable fluorine-19 was traced to the cesium hydroxide used to trap the H18F. Following intracarotid injection, the labeled fluoroethanol was not trapped in the brain, and thus is not a microsphere analog as has been suggested. Tomographic images of the myocardium were obtained using the fluoroethanol as a tracer.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
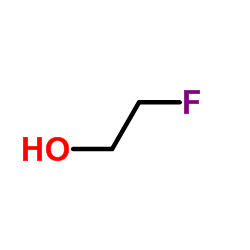 |
2-氟乙醇
CAS:371-62-0 |
C2H5FO |
|
Hydrolytic pathway of 5-fluorouracil in aqueous solutions fo...
2014-09-01 [J. Pharm. Biomed. Anal. 98 , 446-62, (2014)] |
|
Measuring the relative hydrogen-bonding strengths of alcohol...
2015-01-12 [ChemPhysChem 16(1) , 160-8, (2015)] |
|
2-Halogeno-ethanols as an uncoupler of phosphorylation in ra...
1980-05-15 [Experientia 36(5) , 537-9, (1980)] |
|
2-halogeno-ethanols as denaturant of protein. Activity coeff...
1982-01-01 [Fukushima J. Med. Sci. 28(3-4) , 83-92, (1982)] |
|
An automatic method to generate force-field parameters for h...
2003-02-01 [Acta Crystallogr. D Biol. Crystallogr. 59(Pt 2) , 274-89, (2003)] |