Role of the peripheral anionic site on acetylcholinesterase: inhibition by substrates and coumarin derivatives.
Z Radić, E Reiner, P Taylor
文献索引:Mol. Pharmacol. 39(1) , 98-104, (1991)
全文:HTML全文
摘要
Propidium has been demonstrated in previous studies to be a selective ligand for the peripheral anionic site on acetylcholinesterase (EC 3.1.1.7). Its association with this site can be advantageously monitored by direct fluorescent titration. We have measured the ability of acetylcholine, acetylthiocholine, haloxon [di-(2-chloroethyl)3-chloro-4-methylcoumarin-7-ylphosphate] , and a coumarin derivative (3-chloro-7-hydroxy-4-methylcoumarin) to dissociate propidium from the peripheral anionic site of Torpedo californica acetylcholinesterase. Measurements were made by back-titration of propidium after complete inhibition of the active center with diisopropylfluorophosphate. Both acetylcholine and acetylthiocholine show substrate inhibition at high substrate concentrations. The concentrations required for occupation of the peripheral site, as ascertained by competition with propidium, correlated well with the concentration dependence for the kinetics of substrate inhibition. These observations are consistent with substrate inhibition being due to binding of acetylcholine or acetylthiocholine at a peripheral anionic site. Displacement of propidium by haloxon and coumarin indicated that these inhibitors also bind to the peripheral anionic site. The dissociation constants ascertained from peripheral site occupation are in agreement with the constants obtained from inhibition kinetics. Evidence is presented that competition with propidium obtained by direct fluorescence titrations, when combined with inhibition kinetics, provides a more reliable means for ascertaining site selectivity of various inhibitors than does a kinetic analysis alone.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
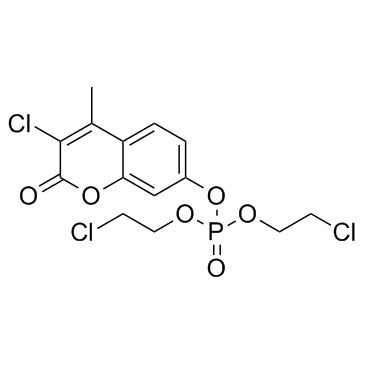 |
Haloxon
CAS:321-55-1 |
C14H14Cl3O6P |
|
Effects of selected organophosphate insecticides on serum ch...
1996-06-01 [Vet. Hum. Toxicol. 38(3) , 196-9, (1996)] |
|
Effect of some anthelmintics on malate dehydrogenase activit...
1986-08-01 [Angew. Parasitol. 27(3) , 175-80, (1986)] |
|
Effect of cambendazole and haloxon on the carbohydrate metab...
1990-05-01 [Angew. Parasitol. 31(2) , 101-5, (1990)] |
|
Haloxon: critical tests of antiparasitic activity in equids.
1981-06-01 [Am. J. Vet. Res. 42(6) , 1043-5, (1981)] |
|
Haloxon poisoning in geese.
1980-12-06 [Vet. Rec. 107(23) , 541, (1980)] |