| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
五水硫酸铜
CAS:7758-99-8 |
|
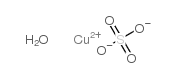 |
五水合硫酸铜(II)
CAS:23254-43-5 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
五水硫酸铜
CAS:7758-99-8 |
|
 |
五水合硫酸铜(II)
CAS:23254-43-5 |