Synthesis of two analogs of a cyclic hexapeptide with disulfide bridge corresponding to bovine prothrombin precursor sequence 18-23. Extent of carboxylation by Vitamin K-Dependent carboxylase.
D H Rich, M Kawai, H L Goodman, J W Suttie
文献索引:Int. J. Pept. Protein Res. 18 , 41, (1981)
全文:HTML全文
摘要
The synthesis of two analogs of sequence 18-23 of bovine prothrombin precursor is described. Hexapeptides Boc-Cys (Acm)-Leu-Glu(OBzl)-Glu(OBzl)-Pro-Cys (Acm)-OBzl and Ac-Cys(Acm-Leu-Glu(OBzl)-Glu(OBzl)-Pro-Cys(Acm)-OMe were synthesized in solution by stepwise addition of Boc-amino acids using dicyclohexylcarbodiimide/N-hydroxybenzotriazole as the coupling reagent. The acetamidomethyl groups were cleaved and oxidized, using iodine in methanol, to the protected cyclic disulfide in 62-69% yield. The O-benzyl groups were removed either by treatment with anhydrous hydrogen fluoride or hydrogen bromide in trifluoroacetic acid to give the cyclic hexapeptide disulfides, R1-Cys-Leu-Glu-Glu-Pro-Cys-OR2 where R1 - H or Ac and R2 = H or CH3. The cyclic hexapeptides were evaluated as substrates for vitamin K-dependent carboxylase. Both peptides are unusually poor substrates for the carboxylase, and each appears to inhibit carboxylation of Phe-Leu-Glu-Glu-Leu, a good substrate for the enzyme.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
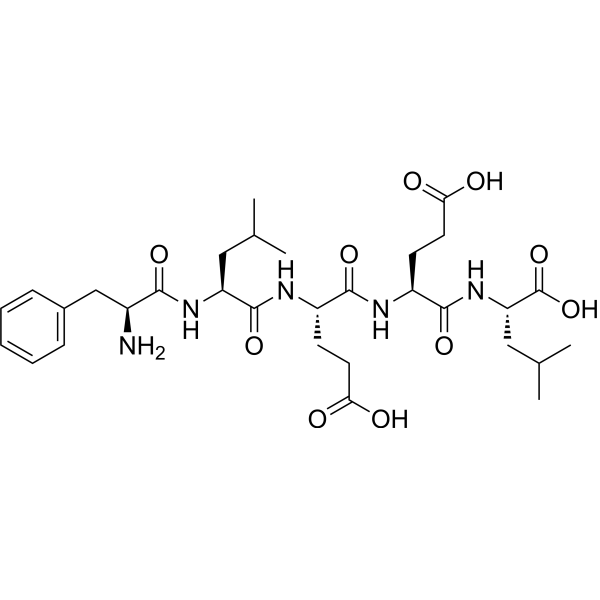 |
H-Phe-Leu-Glu-Glu-Leu-OH
CAS:69729-06-2 |
C31H47N5O10 |
|
Expression and characterization of recombinant vitamin K-dep...
2002-12-01 [Eur. J. Biochem. 269 , 6162-6172, (2002)] |
|
Isolation and partial characterization of a vitamin K-depend...
1987-07-01 [Biochem. J. 245 , 251-255, (1987)] |
|
Vitamin K-dependent carboxylase: purification of the rat liv...
1980-07-01 [Arch. Biochem. Biophys. 202 , 515, (1980)] |
|
Vitamin K-dependent carboxylase. Partial purification and pr...
1982-12-25 [J. Biol. Chem. 257 , 15008-15011, (1982)] |
|
Vitamin K-dependent carboxylase: synthesis of an inhibitor o...
1983-02-07 [FEBS Lett. 152 , 79-82, (1983)] |