| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
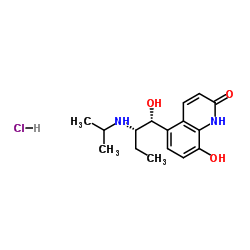 |
盐酸丙卡特罗
CAS:62929-91-3 |
|
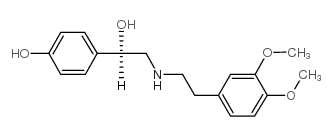 |
R(-)-去甲胺
CAS:71771-90-9 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
盐酸丙卡特罗
CAS:62929-91-3 |
|
 |
R(-)-去甲胺
CAS:71771-90-9 |