Metabolism of dextrorphan by CYP2D6 in different recombinantly expressed systems and its implications for the in vitro assessment of dextromethorphan metabolism.
Linh M Van, Sunil Sarda, Judith A Hargreaves, Amin Rostami-Hodjegan
文献索引:J. Pharm. Sci. 98(2) , 763-71, (2009)
全文:HTML全文
摘要
Cytochrome P450 2D6 (CYP2D6) mediated formation of dextrorphan (DOR) from dextromethorphan (DEX) is widely used as a marker to assess the activity of this enzyme both in vitro and in vivo. The sequential metabolism of DOR during in vitro studies, particularly using recombinant systems (rCYPs) expressing human CYP2D6, is assumed to be negligible. The extent of metabolism was investigated for a range of DEX and DOR concentrations in microsomal preparations from three different rCYPs expressing human CYP2D6 (yeast, Supersomes and Bactosomes) containing 10 pmol of the enzyme. Bactosomes and Supersomes, but not yeast rCYP microsomes, were capable of metabolising DOR to 3-hydroxymorphinan (HYM). Two novel CYP2D6 related metabolites were identified in Bactosomes, and assigned as single hydroxylations in the phenyl rings of DOR and HYM using ion-trap mass spectrometry. Therefore, in rCYP systems with high turn over rate (e.g. Bactosomes) DOR may not be considered as an end product particularly at low concentrations of DEX; leading to an underestimation of true metabolic rate. The results also put further emphasis on the necessity of optimising study conditions when switching between rCYP sources.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
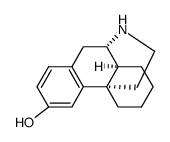 |
(+)-3-HYDROXYMORPHINAN HYDROBROMIDE
CAS:15676-23-0 |
C16H21NO |
|
A validated SIM GC/MS method for the simultaneous determinat...
2009-11-01 [Biomed. Chromatogr. 23(11) , 1131-7, (2009)] |
|
Simultaneous quantification of dextromethorphan and its meta...
2011-01-25 [J. Pharm. Biomed. Anal. 54(2) , 387-94, (2011)] |
|
Validation of a liquid chromatography-mass spectrometry meth...
2005-05-05 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 819(1) , 105-13, (2005)] |
|
New morphinan derivatives with negligible psychotropic effec...
2003-03-07 [Life Sci. 72(16) , 1883-95, (2003)] |
|
Neuropsychotoxicity of abused drugs: potential of dextrometh...
2008-01-01 [J. Pharmacol. Sci. 106(1) , 22-7, (2008)] |