| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
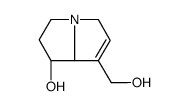 |
倒千里光裂碱
CAS:480-85-3 |
|
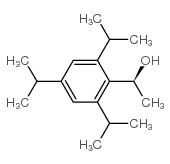 |
(S)-(-)-1-(2,4,6-三异丙基苯基)乙醇
CAS:102225-88-7 |
|
 |
(R)-(+)-1-(2,4,6-三异丙基苯基)乙醇
CAS:181531-14-6 |