Enantioselective hydrolysis of lorazepam 3-acetate by esterases in human and rat liver microsomes and rat brain S9 fraction.
K Liu, F P Guengerich, S K Yang
文献索引:Drug Metab. Dispos. 19(3) , 609-13, (1991)
全文:HTML全文
摘要
Rates of hydrolysis of racemic and enantiomeric lorazepam 3-acetates (LZA) by esterases in human and rat liver microsomes and rat brain S9 fraction were compared. LZA and its hydrolysis product were analyzed by chiral stationary phase HPLC. When rac-LZA was the substrate, the (R)-LZA was hydrolyzed 2.7-fold and 6.8-fold faster than the (S)-LZA by esterases in rat and human liver microsomes, respectively. In contrast, esterases in rat brain S9 fraction were enantioselective toward the (S)-LZA. The specific activities (nmol of LZA hydrolyzed/mg protein/min) of liver microsomes in the hydrolysis of enantiomerically pure (R)-LZA were approximately 210 (rat) and 1330 (human), and in the hydrolysis of enantiomerically pure (S)-LZA were 25 (rat) and 8 (human). The specific activities of rat brain S9 fraction in the hydrolysis of enantiomerically pure (R)-LZA and (S)-LZA were approximately 3 and 6 nmol/mg protein/min, respectively. Results also indicated an enantiomeric interaction in the hydrolysis of rac-LZA; the presence of (R)-LZA stimulated the hydrolysis of (S)-LZA by all esterase preparations, whereas the presence of (S)-LZA stimulated the hydrolysis of (R)-LZA in rat brain S9 fraction and inhibited the hydrolysis of (R)-LZA in rat and human liver microsomes.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
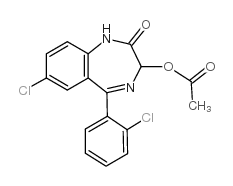 |
7-氯-5-(2-氯苯基)-1,3-二氢-2-氧代-2H-1,4-苯并二氮杂卓-3-基乙酸酯
CAS:2848-96-6 |
C17H12Cl2N2O3 |
|
Stereoselective binding of 3-acetoxy-, and 3-hydroxy-1,4-ben...
1986-01-15 [Biochem. Pharmacol. 35(2) , 263-9, (1986)] |
|
Stereoselective kinetics of warfarin binding to human serum ...
2002-05-15 [Chirality 14(5) , 442-8, (2002)] |
|
Stereoselective allosteric binding interaction on human seru...
1999-01-01 [Chirality 11(2) , 115-20, (1999)] |
|
Recurrent complex partial status epilepticus associated with...
2002-03-01 [Acta Neurol. Belg. 102(1) , 19-20, (2002)] |
|
Application of ultrafiltration and CD spectroscopy for study...
1982-12-15 [Biochem. Biophys. Res. Commun. 109(3) , 851-7, (1982)] |