Study on reactivity and protection of the alpha-hydroxyphosphonate moiety in 5'-nucleotide analogues: formation of the 3'-O-P-C(OH)-C4' internucleotide linkage.
Sárka Králíková, Milena Masojídková, Milos Budĕsínský, Ivan Rosenberg
文献索引:Nucleosides Nucleotides Nucleic Acids 22(3) , 329-47, (2003)
全文:HTML全文
摘要
The recently described epimeric nucleosidyl-5'-C-phosphonates (alpha-hydroxyphosphonates) represent novel nucleotide analogues that can be incorporated into chimeric oligonucleotides by the phosphotriester condensation method. In order to prepare suitable protected monomer(s) we have studied condensation reaction between protected 2'-deoxythymidine and 2'-deoxythymidinyl-5'-C-phosphonate, both as model compounds, in dependence on the nature of the 5'-hydroxyl protecting group. We have found that the O-acetyl group is unstable in the presence of TPSCl or MSNT used as condensing agents for activation of the phosphorus moiety. This instability negatively influences the scope of the condensation process. On the other hand, introduction of the O-methoxycarbonyl group gave excellent results. The O-methoxycarbonyl group does not participate in the condensation process, and its quantitative introduction into the nucleotide analo gues is accomplished using a novel acylating agent, methoxycarbonyl tetrazole.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
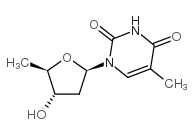 |
5'-脱氧胸苷
CAS:3458-14-8 |
C10H13IN2O4 |
|
Thymidine inhibits the growth-arrest-specific degradation of...
1997-01-17 [J. Mol. Biol. 265(2) , 153-60, (1997)] |
|
Membrane permeation characteristics of 5'-modified thymidine...
1992-05-01 [Mol. Pharmacol. 41(5) , 950-6, (1992)] |
|
Synthesis and HIV-1 reverse transcriptase inhibitor activity...
2003-01-01 [Arzneimittelforschung 53(4) , 266-71, (2003)] |
|
Formation of aminyl radicals on electron attachment to AZT: ...
2010-07-22 [J. Phys. Chem. B 114(28) , 9289-99, (2010)] |
|
A-hydroxyphosphonate oligonucleotides: a promising DNA type?
2003-01-01 [Nucleosides Nucleotides Nucleic Acids 22(5-8) , 1061-4, (2003)] |