Sorption of tylosin on clay minerals.
Qian Zhang, Chen Yang, Weilin Huang, Zhi Dang, Xiaohua Shu
文献索引:Chemosphere 93(9) , 2180-6, (2013)
全文:HTML全文
摘要
The equilibrium sorption of tylosin (TYL) on kaolinite and montmorillonite was measured at different solution pH using batch reactor systems. The results showed that all the sorption isotherms were nonlinear and that the nonlinearity decreased as the solution pH increased for a given clay. At a specific aqueous concentration, the single-point sorption distribution coefficient (KD) of TYL decreased rapidly as the solution pH increased. A speciation-dependent sorption model that accounted for the contributions of the cationic and neutral forms of TYL fit the data well, suggesting that the sorption may be dominated by both ion exchange and hydrophobic interactions. The isotherm data also fit well to a dual mode model that quantifies the contributions of a site-limiting Langmuir component (ion exchange) and a non-specific linear partitioning component (hydrophobic interactions). X-ray diffraction analyses revealed that the interlayers of montmorillonite were expanded due to the uptake of TYL. TYL molecules likely form a monolayer surface coverage.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
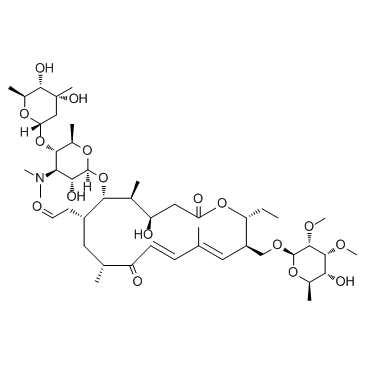 |
泰乐菌素
CAS:1401-69-0 |
C46H77NO17 |
|
Efficacy of in-feed medication with chlortetracycline in a f...
2012-01-01 [Vet. Rec. 171(25) , 645, (2012)] |
|
Effects of copper and aluminum on the adsorption of sulfathi...
2014-01-01 [Environ. Pollut. 184 , 579-85, (2014)] |
|
Extraction of trace tilmicosin in real water samples using i...
2013-01-01 [Water Sci. Technol. 67(8) , 1671-7, (2013)] |
|
Response of intestinal microbiota to antibiotic growth promo...
2013-04-01 [Foodborne Pathog. Dis. 10(4) , 331-7, (2013)] |
|
Validation of two ELISA kits for the screening of tylosin an...
2013-01-01 [Food Addit. Contam. Part A. Chem. Anal. Control. Expo. Risk Assess. 30(1) , 93-109, (2013)] |