Metabolism of phenylhydroquinone by prostaglandin (H) synthase: possible implications in o-phenylphenol carcinogenesis.
P Kolachana, V V Subrahmanyam, D A Eastmond, M T Smith
文献索引:Carcinogenesis 12(1) , 145-9, (1991)
全文:HTML全文
摘要
o-Phenylphenol (OPP) and its sodium salt sodium ortho-phenylphenate (NaOPP) are broad spectrum fungicides and antibacterial agents. Both are urinary bladder and renal carcinogens in the Fischer 344 rat. OPP is converted by mixed-function oxidases in the liver to phenylhydroquinone (PHQ). Since appreciable amounts of prostaglandin (H) synthase (PGS) are found in rat bladder and kidney-medullary papilla, the target sites of OPP- and NaOPP-induced tumors, we hypothesized that a secondary PGS-mediated activation of PHQ to phenylbenzoquinone (PBQ) may occur in the bladder and kidney. We have studied the metabolism of PHQ by PGS in the presence of arachidonic acid and hydrogen peroxide as co-factors. These studies showed that PHQ is indeed metabolized to a product having identical spectral and electrochemical properties to PBQ. The disappearance of PHQ with time was stoichiometric to the formation of PBQ. Less than 10% of PHQ was converted to PBQ in the absence of enzyme, indicating that auto-oxidation may play only a minor role in the conversion of PHQ to PBQ. Similar results were obtained when PGS was replaced with either myeloperoxidase or horseradish peroxidase and hydrogen peroxide as co-factor. These studies suggest that the peroxidative metabolism of PHQ by PGS to the reactive PBQ could play an important role in OPP-induced urinary bladder and kidney carcinogenesis in rats.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
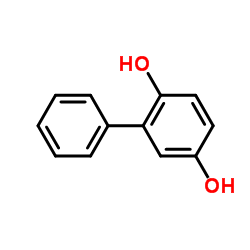 |
2,5-二羟基联苯
CAS:1079-21-6 |
C12H10O2 |
|
Cytotoxic effects of phenyl-hydroquinone and some hydroquino...
1992-09-25 [Biochem. Pharmacol. 44(6) , 1059-65, (1992)] |
|
Tuning surface hydrophilicity/hydrophobicity of hydrocarbon ...
2016-03-15 [J. Colloid. Interface Sci. 466 , 168-77, (2016)] |
|
The inhibition of phenylhydroquinone-induced oxidative DNA c...
2000-02-01 [Biol. Pharm. Bull. 23(2) , 199-203, (2000)] |
|
Metabolites of the biocide o-phenylphenol generate oxidative...
2000-01-01 [Arch. Toxicol. 73(10-11) , 607-10, (2000)] |
|
DNA adduct formation by o-phenylphenol metabolite in vivo an...
1992-08-01 [Carcinogenesis 13(8) , 1469-73, (1992)] |