Carbon isotope fractionation in the decarboxylation of phenylpropiolic acid in hydrogen donating media.
M Zieliński, A Zielińska, H Papiernik-Zielińska, N Ogrinc, I Kobal
文献索引:Isotopes Environ. Health Stud. 37(3) , 239-52, (2001)
全文:HTML全文
摘要
13C kinetic isotope effect (KIE) in the decarboxylation of phenylpropiolic acid (PPA) in tetralin medium (Tn) has been determined at 409-432 K and found to be of magnitude similar to the 13C KIE observed in the decarboxylation of malonic acid where the rupture of the C-C bond is the rate determining step. 13C KIE equals 1.0318/at 136 degrees C in the decarboxylation of PPA in Tn medium. Intramolecular 13C KIE in the decarboxylation of malonic acid equals 1.0316 at this temperature. Thus it has been shown that the nearly "full" 13C KIE can be achieved by providing the excess hydrogen to Calpha of PPA (or to triple acetylene bond) using not only strong mineral acids as the source of protons but also by carrying out the decarboxylation in organic medium like tetralin. A mechanism of decarboxylation of PPA in Tn is suggested.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
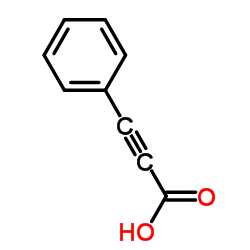 |
苯丙炔酸
CAS:637-44-5 |
C9H6O2 |
|
Structure-based design and mechanisms of allosteric inhibito...
2013-06-11 [Proc. Natl. Acad. Sci. U. S. A. 110(24) , 9728-33, (2013)] |
|
Inactivation of peptidylglycine α-hydroxylating monooxygenas...
2016-08-01 [J. Enzyme Inhib. Med. Chem. 31 , 551-62, (2016)] |
|
Time domain para hydrogen induced polarization
2012-06-01 [Solid State Nucl. Magn. Reson. 43-44 , 14-21, (2012)] |
|
Metabolic reprogramming in plant innate immunity: the contri...
2004-04-01 [Immunol. Rev. 198 , 267-84, (2004)] |
|
[Allelopathy of grape root aqueous extracts].
2010-07-01 [Ying Yong Sheng Tai Xue Bao 21(7) , 1779-84, (2010)] |