Synthesis, structures, spectroscopic and electrochemical properties of dinitrosyl iron complexes with bipyridine, terpyridine, and 1,10-phenathroline.
Rongming Wang, Ximeng Wang, Eric B Sundberg, Phuongmei Nguyen, Gian Paola G Grant, Chaitali Sheth, Qiang Zhao, Steve Herron, Katherine A Kantardjieff, Lijuan Li
文献索引:Inorg. Chem. 48(20) , 9779-85, (2009)
全文:HTML全文
摘要
Three new dinitrosyl iron complexes LFe(NO)(2) (L = 2,2'-bipyridine (bipy) (1), 2,2',2''-terpyridine (terpy) (2) and 1,10-phenathroline (phen) (3)) were synthesized by the reaction of Fe(NO)(2)(CO)(2) with corresponding ligands in tetrahydrofuran. Complexes 1-3 were studied using IR, UV-vis, MS, NMR, and electrochemical techniques. Complexes 1 and 2 were also characterized using single crystal X-ray diffraction analysis. IR spectra of complexes 1-3 display two strong characteristic NO stretching frequencies (nu(NO)) in the region reflecting donor properties of the ligands. Cyclic voltammetry studies show two quasi-reversible one-electron reductions for all complexes. Electrochemical investigations using different concentrations show that an irreversible one-electron reduction at -1.85 V for complex 2 and -1.80 V for complex 3 are from solvated species. Single-crystal X-ray structural analysis reveals that complex 1 crystallizes in the triclinic P1 space group and the asymmetric unit consists of one Fe(NO)(2)(bipy) molecule with the two NO groups located on two sides of Fe(bipy) plane. Complex 2 crystallizes in monoclinic P21/n space group, and the asymmetric unit contains one Fe(NO)(2)(terpy) molecule, in which the NO groups are located on two sides of the plane consisted of Fe and two coordinated pyridyl rings, but almost parallel to the uncoordinated pyridyl ring. The crystal packings of both complexes 1 and 2 show intermolecular H-bonding and strong pi-pi stacking interactions.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
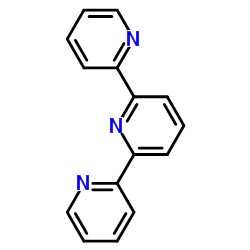 |
2,2':6',2''-三吡啶
CAS:1148-79-4 |
C15H11N3 |
|
Thousands of chemical starting points for antimalarial lead ...
2010-05-20 [Nature 465 , 305-10, (2010)] |
|
Metal complexes with superoxide dismutase-like activity as c...
2006-12-01 [Bioorg. Med. Chem. Lett. 16 , 5982-7, (2006)] |
|
In silico activity profiling reveals the mechanism of action...
2008-07-01 [Proc. Natl. Acad. Sci. U. S. A. 105 , 9059-64, (2008)] |
|
Rapid and sensitive amino-acid sequencing of cloning Thermus...
2004-01-01 [J. Proteome Res. 3(5) , 983-7, (2004)] |
|
Synthesis of 2,6-diaryl-substituted pyridines and their anti...
2008-04-01 [Eur. J. Med. Chem. 43 , 675-82, (2008)] |