Efficient new ribozyme mimics: direct mapping of molecular design principles from small molecules to macromolecular, biomimetic catalysts.
W C Putnam, A T Daniher, B N Trawick, J K Bashkin
文献索引:Nucleic Acids Res. 29(10) , 2199-204, (2001)
全文:HTML全文
摘要
Dramatic improvements in ribozyme mimics have been achieved by employing the principles of small molecule catalysis to the design of macromolecular, biomimetic reagents. Ribozyme mimics derived from the ligand 2,9-dimethylphenanthroline (neocuproine) show at least 30-fold improvements in efficiency at sequence-specific RNA cleavage when compared with analogous o-phenanthroline- and terpyridine-derived reagents. The suppression of hydroxide-bridged dimers and the greater activation of coordinated water by Cu(II) neocuproine (compared with the o-phenanthroline and terpyridine complexes) better allow Cu(II) to reach its catalytic potential as a biomimetic RNA cleavage agent. This work demonstrates the direct mapping of molecular design principles from small-molecule cleavage to macromolecular cleavage events, generating enhanced biomimetic, sequence-specific RNA cleavage agents.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
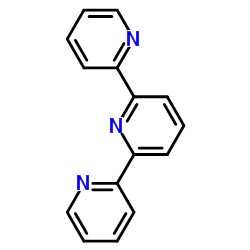 |
2,2':6',2''-三吡啶
CAS:1148-79-4 |
C15H11N3 |
|
Thousands of chemical starting points for antimalarial lead ...
2010-05-20 [Nature 465 , 305-10, (2010)] |
|
Metal complexes with superoxide dismutase-like activity as c...
2006-12-01 [Bioorg. Med. Chem. Lett. 16 , 5982-7, (2006)] |
|
In silico activity profiling reveals the mechanism of action...
2008-07-01 [Proc. Natl. Acad. Sci. U. S. A. 105 , 9059-64, (2008)] |
|
Rapid and sensitive amino-acid sequencing of cloning Thermus...
2004-01-01 [J. Proteome Res. 3(5) , 983-7, (2004)] |
|
Synthesis of 2,6-diaryl-substituted pyridines and their anti...
2008-04-01 [Eur. J. Med. Chem. 43 , 675-82, (2008)] |