Hydroxylating activity of tyrosinase and its dependence on hydrogen peroxide.
M Jiménez, F García-Carmona
文献索引:Arch. Biochem. Biophys. 373(1) , 255-60, (2000)
全文:HTML全文
摘要
The aim of this work was to study the hydroxylation of N, N-dimethyltyramine (DMTA) by tyrosinase in the presence of hydrogen peroxide, a reaction that does not take place without the addition of the hydrogen peroxide. Some properties of this hydroxylating activity are analyzed. The kinetic parameters of mushroom tyrosinase toward hydrogen peroxide (K(m) = 0.5 mM, V(m) = 11 microM/min, V(m)/K(m) = 2.2 x 10(-2) min(-1)) and toward DMTA (K(m) = 0.3 mM, V(m) = 4.8 microM/min, V(m)/K(m) = 16 x 10(-2) min(-1)) were evaluated. There was a lag period, which was similar to the characteristic lag of monophenolase activity at the expense of molecular oxygen. The length of this lag phase decreased with increasing hydrogen peroxide concentration, and disappeared at approximately 0.5 mM H(2)O(2). However, the lag was longer with higher DMTA concentrations. The pH optimum range for this hydroxylating activity was 6.0 to 7.0. The lag also varied with pH, increasing at pH values higher than 6.7. The presence of hydrogen peroxide is necessary for the oxidation of DMTA, as is the presence of active enzyme since the reaction was completely inhibited when selective tyrosinase inhibitors were added.Copyright 2000 Academic Press.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
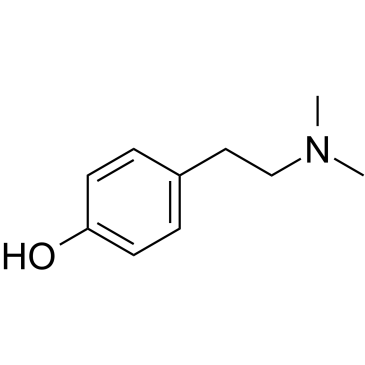 |
大麦芽碱
CAS:539-15-1 |
C10H15NO |
|
Development of immunoassays for tyramine and tryptamine toxi...
2000-01-01 [J. Agric. Food Chem. 48(1) , 27-32, (2000)] |
|
Some hematological and histopathological effects of the alka...
1980-01-01 [Toxicology 18(2) , 125-31, (1980)] |
|
Rapid formation of N-nitrosodimethylamine from gramine, a na...
1984-01-01 [IARC Sci. Publ. (57) , 337-46, (1984)] |
|
N-nitrosodimethylamine precursors in malt.
1982-01-01 [IARC Sci. Publ. (41) , 71-80, (1982)] |
|
Deamination of hordenine by monoamine oxidase and its action...
1989-06-01 [J. Pharm. Pharmacol. 41(6) , 421-3, (1989)] |