Synthesis of [3,4-(13)c(2)]-enriched bile salts as NMR probes of protein-ligand interactions.
Gregory P Tochtrop, Gregory T DeKoster, David P Cistola, Douglas F Covey
文献索引:J. Org. Chem. 67(19) , 6764-71, (2002)
全文:HTML全文
摘要
Synthetic methodology that allows for incorporation of isotopic carbon at the C-3 and C-4 positions of bile salts is reported. Three [3,4-(13)C(2)]-enriched bile salts were synthesized from either deoxycholic or lithocholic acid. The steroid 3alpha-OH group was oxidized and the A-ring was converted into the Delta(4)-3-ketone. The C-24 carboxylic acid was next converted into the carbonate group and selectively reduced to the alcohol in the presence of the A-ring enone. Following protection of the 24-OH group, the Delta(4)-3-ketone was converted into the A-ring enol lactone. Condensation of the enol lactone with [1,2-(13)C(2)]-enriched acetyl chloride and subsequent Robinson annulation afforded a [3,4-(13)C(2)]-enriched Delta(4)-3-ketone that was subsequently converted back into a 3alpha-hydroxy-5beta-reduced bile steroid. C-7 hydroxylation, when necessary, was achieved via conversion of the Delta(4)-3-ketone into the corresponding Delta(4,6)-dien-3-one, epoxidation of the Delta(6)-double bond, and hydrogenolysis/hydrogenation of the 5,6-epoxy enone system. The [3,4-(13)C(2)]-enriched bile salts were subsequently complexed to human ileal bile acid binding protein (I-BABP), and (1)H-(13)C HSQC spectra were recorded to show the utility of the compounds for investigating the interactions of bile acids with I-BABP.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
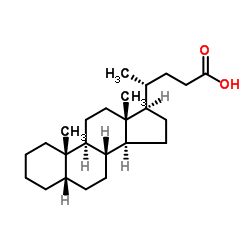 |
5β-胆烷酸
CAS:546-18-9 |
C24H40O2 |
|
An efficient synthesis of 4 beta- and 6 alpha-hydroxylated b...
1993-02-01 [Steroids 58(2) , 52-8, (1993)] |
|
Calcium- and voltage-gated potassium (BK) channel activators...
2012-10-01 [ChemMedChem 7(10) , 1784-92, (2012)] |
|
Cholanic acids determined in commercial drugs by means of a ...
1993-01-01 [J. Pharm. Biomed. Anal. 11(11-12) , 1207-14, (1993)] |
|
Microwave-induced organic reactions of bile acids: esterific...
1995-06-01 [Steroids 60(6) , 453-7, (1995)] |
|
Synthesis of 7- and 12-hydroxy- and 7,12-dihydroxy-3-keto-5 ...
1993-11-01 [Steroids 58(11) , 524-6, (1993)] |