Alosetron for severe diarrhea-predominant irritable bowel syndrome: improving patient outcomes.
Scott Bleser
文献索引:Curr. Med. Res. Opin. 27(3) , 503-12, (2011)
全文:HTML全文
摘要
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder often diagnosed and managed by primary care physicians (PCPs). Despite the high prevalence of IBS, symptom severity is often underappreciated and inadequately managed. The goal of this review is to discern IBS treatment gaps and identify opportunities for improving its management in the primary care setting, as well as describe the most current clinical experience with alosetron, a targeted treatment for severe diarrhea-predominant IBS (IBS-D) in women.PubMed was searched for English language articles using combinations of the following key words: 'irritable bowel syndrome,' 'diarrhea-predominant irritable bowel syndrome,' 'diagnosis,' 'guidelines,' 'general practice,' 'primary care,' 'quality of life,' 'burden,' 'prevalence,' 'patient satisfaction,' 'patient survey,' 'severe,' 'severity,' and 'alosetron.'Establishing the diagnosis of IBS in primary care represents a clinical challenge for many healthcare professionals. While many patients seek care for IBS symptoms in the primary care setting, evidence shows that PCPs are often unaware of established diagnostic criteria for IBS. Establishing the severity of IBS is also problematic, given the lack of consensus guidelines defining severe IBS, which in turn complicates treatment decisions. Severe IBS is often inferred after inadequate response to conventional agents; the level of disease impact on quality of life and patient functioning also defines severity. The selective 5-HT(3) antagonist alosetron has been shown to provide improvement across multiple symptom domains, and the incidence of adverse events continues to be low since the implementation of the Prescribing Program for Lotronex. Alosetron is the only agent approved by the US Food and Drug Administration for treatment of severe IBS-D in women.PCPs often are required to evaluate and treat suspected IBS. The diagnosis and management of IBS in the primary care setting could be optimized through the use of published diagnostic criteria, adequate assessment of symptom severity, and a thorough knowledge of the therapeutic agents that have provided evidence of effectiveness. In the subset of women who suffer from IBS-D, targeted serotonergic therapy with alosetron has been shown to provide symptom relief across multiple domains, including improvement of the patient's quality of life.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
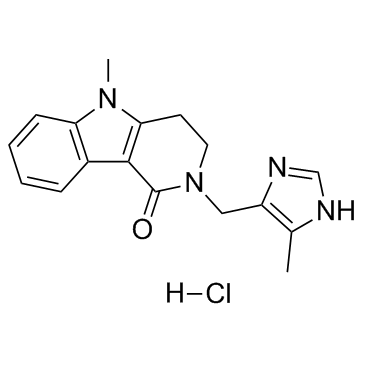 |
盐酸阿洛司琼
CAS:122852-69-1 |
C17H19ClN4O |
|
In silico binary classification QSAR models based on 4D-fing...
2010-07-26 [J. Chem. Inf. Model. 50(7) , 1304-18, (2010)] |
|
Quantitative benefit-risk assessment using only qualitative ...
2012-01-01 [Med. Decis. Making 32(6) , E1-15, (2012)] |
|
Clinical trials in irritable bowel syndrome: a review.
2013-03-01 [Rev. Recent Clin. Trials 8(1) , 9-22, (2013)] |
|
Quality of life in patients with irritable bowel syndrome.
2011-08-01 [J. Clin. Gastroenterol. 45 Suppl , S98-101, (2011)] |
|
Efficacy and tolerability of alosetron for the treatment of ...
2008-05-01 [Clin. Ther. 30(5) , 884-901, (2008)] |