Alosetron for severe diarrhea-predominant irritable bowel syndrome: safety and efficacy in perspective.
James H Lewis
文献索引:Expert Rev. Gastroenterol. Hepatol. 4(1) , 13-29, (2010)
全文:HTML全文
摘要
Irritable bowel syndrome affects 5-10% of North Americans, with an estimated one-third having a diarrhea-predominant form. Alosetron hydrochloride (Lotronex) is a serotonin receptor type 3 antagonist approved in early 2000 for use in women with diarrhea-predominant irritable bowel syndrome (IBS-D). Initial use was widespread, but infrequent serious adverse events of ischemic colitis and severe constipation-related complications prompted alosetron's voluntary withdrawal from the US market in November 2000. Unprecedented public request prompted its reintroduction in 2002 under a Risk Management Plan, including a more restricted indication and a Prescribing Program for Lotronex. Despite these measures, the use of alosetron has been very limited since its reintroduction. Possible deterrents to its use include concerns over safety and the possible medical-legal implications raised by the Risk Management Plan. It is also possible that changes in the natural history and/or diagnosis of IBS-D have reduced the target population. Given the unique regulatory history of alosetron, these issues continue to engender controversy. This article profiles these concerns and reviews the pharmacology, clinical efficacy and safety, and post-marketing experience with alosetron. Myths and misconceptions related to alosetron use, or lack thereof, are addressed to provide the reader with the evidence needed to make informed treatment decisions for their female patients with severe IBS-D.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
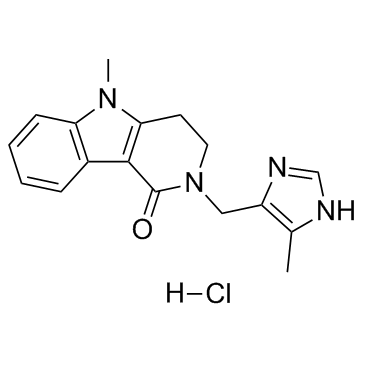 |
盐酸阿洛司琼
CAS:122852-69-1 |
C17H19ClN4O |
|
In silico binary classification QSAR models based on 4D-fing...
2010-07-26 [J. Chem. Inf. Model. 50(7) , 1304-18, (2010)] |
|
Quantitative benefit-risk assessment using only qualitative ...
2012-01-01 [Med. Decis. Making 32(6) , E1-15, (2012)] |
|
Clinical trials in irritable bowel syndrome: a review.
2013-03-01 [Rev. Recent Clin. Trials 8(1) , 9-22, (2013)] |
|
Quality of life in patients with irritable bowel syndrome.
2011-08-01 [J. Clin. Gastroenterol. 45 Suppl , S98-101, (2011)] |
|
Efficacy and tolerability of alosetron for the treatment of ...
2008-05-01 [Clin. Ther. 30(5) , 884-901, (2008)] |