Base-pairing properties of the oxidized cytosine derivative, 5-hydroxy uracil.
Varatharasa Thiviyanathan, Anoma Somasunderam, David E Volk, Tapas K Hazra, Sankar Mitra, David G Gorenstein
文献索引:Biochem. Biophys. Res. Commun. 366(3) , 752-7, (2008)
全文:HTML全文
摘要
The most abundant base-substitution mutation resulting from oxidative damage to DNA is the GC to AT transition mutation. 5-hydroxyuracil (5-OHU), produced by the oxidative deamination of cystosine, has been established as the major chemical precursor for this most abundant transition mutation. Results from NMR spectroscopy and UV melting experiments show that 5-OHU would form the most stable pair with G, and the least stable pair with C. The hydroxyl group in the 5th position of the 5-OHU residue may play a role in increasing the stability of the 5-OHU:G pair over the normal Watson-Crick pair, the 5-OHU:A. The 5-OHU:C base pair would be least stable, and would destabilize the base-stacking in the duplex. Our results explain why certain DNA polymerases preferentially incorporate G opposite to 5-OHU over A and why C does not get incorporated against 5-OHU during DNA replication in vivo.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
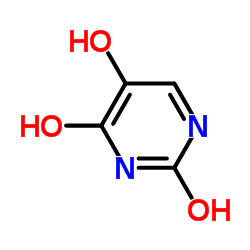 |
5-羟基尿嘧啶
CAS:496-76-4 |
C4H4N2O3 |
|
Hydrolytic pathway of 5-fluorouracil in aqueous solutions fo...
2014-09-01 [J. Pharm. Biomed. Anal. 98 , 446-62, (2014)] |
|
Mutational analysis of the damage-recognition and catalytic ...
2004-01-01 [Nucleic Acids Res. 32(17) , 5291-302, (2004)] |
|
Base-promoted reaction of 5-hydroxyuracil derivatives with p...
2010-09-17 [Org. Lett. 12(18) , 4130-3, (2010)] |
|
NEIL1 is the major DNA glycosylase that processes 5-hydroxyu...
2007-04-03 [Biochemistry 46(13) , 4158-63, (2007)] |
|
Substrate specific stimulation of NEIL1 by WRN but not the o...
2010-06-04 [DNA Repair (Amst.) 9(6) , 636-42, (2010)] |