Synthesis and biological evaluation of new bischromone derivatives with antiproliferative activity.
Agata Szulawska-Mroczek, Marta Szumilak, Malgorzata Szczesio, Andrzej Olczak, Ryszard B Nazarski, Wieslawa Lewgowd, Malgorzata Czyz, Andrzej Stanczak
文献索引:Arch. Pharm. (Weinheim) 346(1) , 34-43, (2013)
全文:HTML全文
摘要
The synthesis of new bischromone derivatives (4a-c and 5a-c) as potential anticancer drugs is described. The difference in the reactivity between 4-oxo-4H-chromene-3-carboxylic acid 2 (or its methyl ester 3) and 4-oxo-4H-chromene-3-carbonyl chloride 1 with three different polyamines: 3,3'-diamino-N-methyldipropylamine (a), 1,4-bis(3-aminopropyl)piperazine (b), 4,9-dioxa-1,12-dodecanediamine (c) resulted in the formation of two different groups of products, compounds 4a-c and 5a-c, designed in agreement with the bisintercalators' structural requirements. The transformation of 4-oxo-4H-chromene-3-carboxylic acid into 2H-chromene-2,4(3H)-diones (5) was confirmed by the NMR and XRD experiments. Compounds 4a and 5a were evaluated in vitro in the highly aggressive melanoma cell line A375. An enhanced induction of apoptosis and cell cycle arrest clearly revealed that compound 5a was more potent than 4a. Compound 5a was also more active in diminishing the adhesive potential of melanoma cells. Current studies support the notion that small changes in the three-dimensional structure of molecules might have a substantial impact on their biological activity.Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
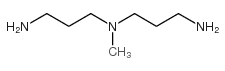 |
N'N-双(3-氨丙基)甲胺
CAS:105-83-9 |
C7H19N3 |
|
Tumor Repression of VCaP Xenografts by a Pyrrole-Imidazole P...
2015-01-01 [PLoS ONE 10 , e0143161, (2015)] |
|
Generation of a focused poly(amino ether) library: polymer-m...
2012-01-01 [Theranostics 2 , 1160-73, (2013)] |
|
Visualization of acidic organelles in intact cells by electr...
1984-08-01 [Proc. Natl. Acad. Sci. U. S. A. 81(15) , 4838-42, (1984)] |
|
Synthesis andin vitrobiological evaluation of new polyamine ...
2010-01-01 [Eur. J. Med. Chem. 45(12) , 5744-51, (2010)] |
|
Targeting of mitochondria-endoplasmic reticulum by fluoresce...
2011-01-01 [PLoS ONE 6 , e27078, (2011)] |