| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
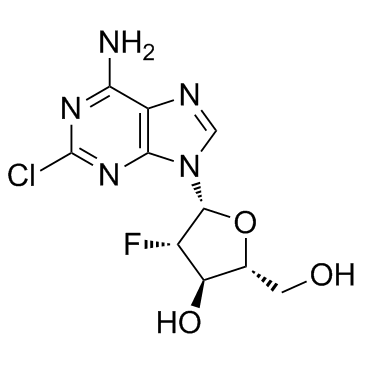 |
克罗拉滨
CAS:123318-82-1 |
|
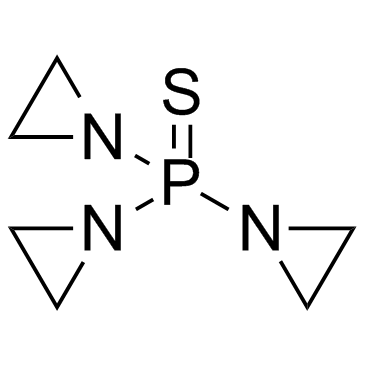 |
塞替派
CAS:52-24-4 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
克罗拉滨
CAS:123318-82-1 |
|
 |
塞替派
CAS:52-24-4 |