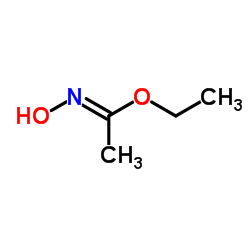
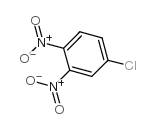
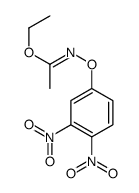
|
~73% |
|
~74% |
|
~49% |
|
~% |
|
~% |
|
~96% |
|
~98% |
|
~60% |
|
~96% |
|
~82% |
|
~% |
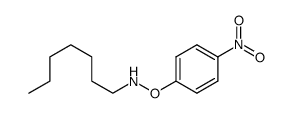
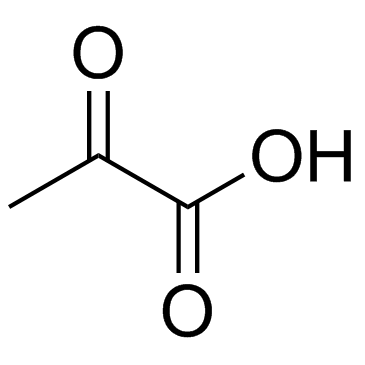
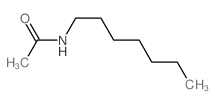
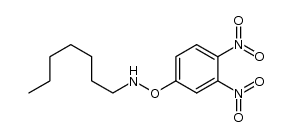
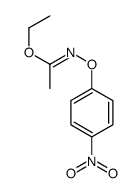


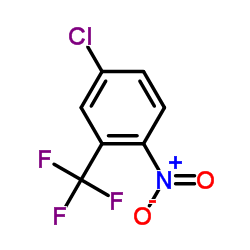

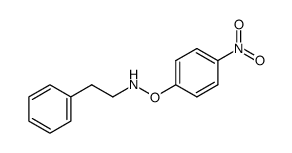
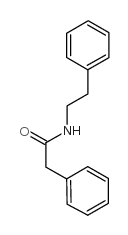
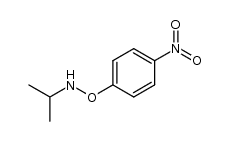
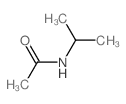
![O-[4-nitro-3-(trifluoromethyl)phenyl]-N-phenethylhydroxylamine结构式](https://image.chemsrc.com/caspic/041/1415337-76-6.png)