| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
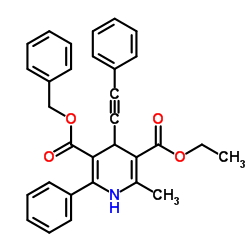 |
乙酰胆碱酯酶 来源于苍蝇头部
CAS:9000-81-1 |
|
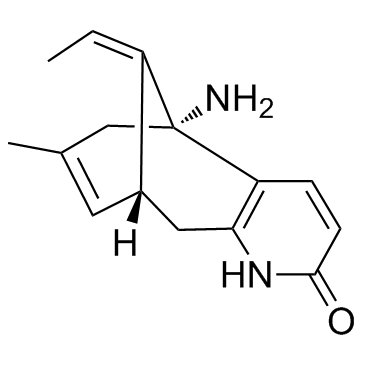 |
(-)-石杉碱甲
CAS:102518-79-6 |