Spontaneous formation of core/shell bimetallic nanoparticles: a calorimetric study.
Naoki Toshima, Masao Kanemaru, Yukihide Shiraishi, Yoshikata Koga
文献索引:J. Phys. Chem. B 109(34) , 16326-31, (2005)
全文:HTML全文
摘要
We showed recently that low entropy core/shell structured nanoparticles form spontaneously from the physical mixture of a dispersion of Ag nanoparticles and that of another noble metal (Rh, Pd, or Pt) at room temperature. Here we use isothermal titration calorimetry (ITC) and show that the initial step of such a spontaneous process is strongly exothermic. When the alcohol dispersion of poly(N-vinyl-2-pyrrolidone) (PVP)-protected Rh nanoparticles (average diameter 2.3 nm) was titrated into the alcoholic dispersion of PVP-protected Ag nanoparticles, a strong exothermic enthalpy change, DeltaH, was observed: DeltaH = -908 kJ/mol for Ag(S) nanoparticle (average diameter 10.8 nm) and -963 kJ/mol for Ag(L) nanoparticles (average diameter 22.5 nm). The strength of interaction increases in the order of Rh/Ag > Pd/Ag > Pt/Ag. This strong exothermic interaction is considered as a driving force to from low entropy bimetallic nanoparticles by simple mixing of two kinds of monometallic nanoparticles. We show also that exothermic interactions occur between a pair of noble metal nanoparticles themselves by using ITC.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
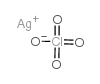 |
高氯酸银
CAS:7783-93-9 |
AgClO4 |
|
Photochemical synthesis of silver particles in Tween 20/wate...
2009-01-01 [J. Colloid. Interface Sci. 339(2) , 373-81, (2009)] |
|
Silver-plated vitamins: a method of detecting tocopherols an...
1998-10-15 [Anal. Chem. 70(20) , 4394-400, (1998)] |
|
Analysis of the response of Candida albicans cells to Silver...
2010-05-01 [Med. Mycol 48(3) , 498-505, (2010)] |
|
In vitro anti-tumour effect of 1,10-phenanthroline-5,6-dione...
2006-12-01 [Chem. Biol. Interact. 164(1-2) , 115-25, (2006)] |
|
Glycosylation via Cp2ZrCl2/AgClO4-mediated activation of ano...
2001-11-16 [J. Org. Chem. 66(23) , 7910-4, (2001)] |