Poly(dA).poly(dT) forms very stable nucleosomes at higher temperatures.
H L Puhl, M J Behe
文献索引:J. Mol. Biol. 245(5) , 559-67, (1995)
全文:HTML全文
摘要
The synthetic polymer poly(dA).poly(dT) was long thought to be refractory to nucleosome formation. Several years ago our laboratory demonstrated that the polymer could be mixed with authentic nucleosomes in a low-salt exchange procedure to form a nucleoprotein complex that behaved in a manner identical with that of nucleosomes. Competitive exchange assays at 37 degrees C showed that the homopolymer reconstituted about as well as heterogenous-sequence DNA. However, studies by other laboratories have shown that the conformation of poly(dA).poly(dT) depends on temperature; the polymer converts from its well-known, atypical structure, found at ambient temperature, to a conformation more closely resembling a canonical B form as temperature is increased. We have measured the ability of the homopurine.homopyrimidine to form nucleosomes as a function of temperature. It is seen that poly(dA).poly(dT) forms nucleosomes more strongly as the temperature of the exchange mixture is increased, so that poly(dA).(dT) outcompetes heterogeneous-sequence DNA for histones at elevated temperatures.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
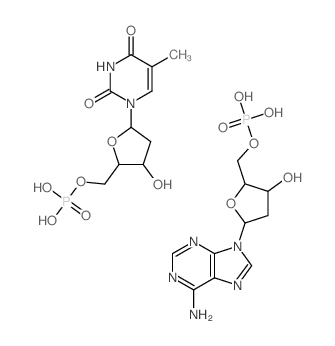 |
聚脱氧腺苷酸.聚胸苷酸 钠盐
CAS:86834-22-2 |
C20H29N7O14P2 |
|
Homopolymer tract length dependent enrichments in functional...
2004-12-14 [BMC Genomics 5 , 95, (2004)] |
|
Energetics of binding the mammalian high mobility group prot...
2005-09-23 [J. Mol. Biol. 352(3) , 629-45, (2005)] |
|
Investigation of DNA binding modes for a symmetrical cyanine...
2001-06-01 [J. Biomol. Struct. Dyn. 18(6) , 844-56, (2001)] |
|
[Hydration and stability of the double helical complex poly(...
1994-01-01 [Biofizika 39(4) , 628-36, (1994)] |
|
Dispersion of meso-tetrakis(N-methylpyridinium-4-yl)porphyri...
2005-11-30 [Biochim. Biophys. Acta 1726(3) , 287-92, (2005)] |