Agents for the treatment of overactive detrusor. V. Synthesis and inhibitory activity on detrusor contraction of N-tert-butyl-4,4-diphenyl-2-cyclopentenylamine.
K Take, K Okumura, K Tsubaki, K Taniguchi, T Terai, Y Shiokawa
文献索引:Chem. Pharm. Bull. 44(10) , 1858-64, (1996)
全文:HTML全文
摘要
N-tert-Butyl-4,4-diphenyl-2-cyclopentenylamine ((+/-)-3) was designed to restrict the conformation of terodiline 1 and was synthesized in a 6-step approach starting with diphenylacetaldehyde (10) or in a 4-step approach starting with 2,2-diphenyl-4-pentenoic acid (17). Using di-p-toluoyltartaric acid as a resolving agent, the synthetic (+/-)-3 was resolved into its optically pure forms, (-)- and (+)-3. The (-)-enantiomer (-)-3.HCl (FK584) showed about ten times more potent inhibitory activity on urinary bladder rhythmic contraction in rats (ED30 = 0.18 mg/kg, i.v.) than terodiline (ED30 = 1.9 mg/kg, i.v.), while the (+)-enantiomer (+)-3.HCl showed no inhibitory activity at 1.0 mg/kg i.v. Compound (-)-3.HCl (FK584) has pharmacological properties similar to those of terodiline, as evaluated by in vitro assay and is currently in clinical development for the treatment of overactive detrusor.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
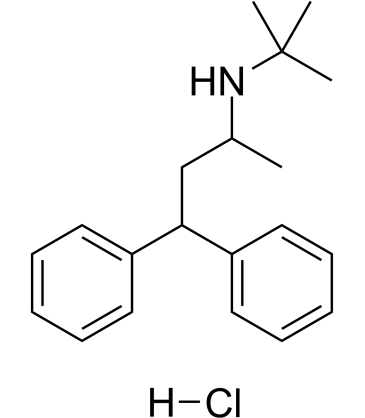 |
Terodiline hydrochloride
CAS:7082-21-5 |
C20H28ClN |
|
Action potentials, contraction, and membrane currents in gui...
1999-09-01 [J. Pharmacol. Exp. Ther. 290(3) , 1417-26, (1999)] |
|
Comparison of the effects of NS-21 and terodiline on the QTc...
1998-01-01 [Gen. Pharmacol. 30(1) , 137-42, (1998)] |
|
CYP2D6 and CYP2C19 genotypes of patients with terodiline car...
2000-07-01 [Br. J. Clin. Pharmacol. 50(1) , 77-80, (2000)] |
|
Inhibition of cardiac inward-rectifier K+ current by terodil...
1999-07-01 [Eur. J. Pharmacol. 370(3) , 319-27, (1999)] |
|
Pharmacokinetic/pharmacodynamic assessment of the effects of...
2001-01-01 [Xenobiotica 31(8-9) , 633-50, (2001)] |