Synthesis of lacto-N-neotetraose and lacto-N-tetraose using the dimethylmaleoyl group as amino protective group.
M R Aly, I Ibrahim el-S, H Ashry el-S, R R Schmidt
文献索引:Carbohydr. Res. 316(1-4) , 121-32, (1999)
全文:HTML全文
摘要
The disaccharide donor O-[2,3,4,6-tetra-O-acetyl-beta-D- galactopyranosyl)-(1-->4)-3,6-di-O-benzyl-2-deoxy-2-dimethylmaleimido - alpha,beta-D-glucopyranosyl] trichloroacetimidate (7) was prepared by reacting O-(2,3,4,6-tetra-O-acetyl- alpha-D-galactopyranosyl) trichloroacetimidate with tert-butyldimethylsilyl 3,6-di-O-benzyl-2-deoxy-2- dimethylmaleoylamido-glucopyranoside to give the corresponding disaccharide 5. Deprotection of the anomeric center and then reaction with trichloroacetonitrile afforded 7. Reaction of 7 with 3'-O-unprotected benzyl (2,4,6-tri-O-benzyl-beta-D-galactopyranosyl)- (1-->4)-2,3,6-tri-O-benzyl-beta-D-glucopyranoside (8) as acceptor afforded the desired tetrasaccharide benzyl (2,3,4,6-tetra-O-acetyl-beta-D-galactopyranosyl)-(1-->4)-(3,6-di-O- benzyl-2-deoxy-2-dimethylmaleimido-beta-D-glucopyranosyl)-(1-->3)- (2,4,6- tri-O-benzyl-beta-D-galactopyranosyl)-(1-->4)-2,3,6-tri-O-benzyl-beta-D- glucopyranoside. Replacement of the N-dimethylmaleoyl group by the acetyl group, O-debenzylation and finally O-deacetylation gave lacto-N-neotetraose. Similarly, reaction of O-[(2,3,4,6-tetra-O-acetyl-beta- D-galactopyranosyl)-(1-->3)-4,6-O-benzylidene-2-deoxy-2-dimethylmalei mido- alpha,beta-D-glycopyranosyl] trichloroacetimidate as donor with 8 as acceptor afforded the desired tetrasaccharide benzyl (2,3,4,6-tetra-O-acetyl-beta-D- galactopyranosyl)-(1-->3)-(4,6-benzylidene-2-deoxy-2-dimethylmaleimid o- beta-D-glucopyranosyl)-(1-->3)-(2,4,6-tri-O-benzyl-beta-D-galactopyranos yl)- (1-->4)-2,3,6-tri-O-benzyl-beta-D-glucopyranoside. Removal of the benzylidene group, replacement of the N-dimethylmaleoyl group by the acetyl group and then O-acetylation afforded tetrasaccharide intermediate 15, which carries only O-benzyl and O-acetyl protective groups. O-Debenzylation and O-deacetylation gave lacto-N-tetraose (1). Additionally, known tertbutyldimethylsilyl (2,3,4,6-tetra-O-acetyl-beta-D-galactopyranosyl)-(1-->3)-4,6-O-benzylide ne- 2-deoxy-2-dimethylmaleimido-beta-D-glucopyranoside was transformed into O-[2,3,4,6-tetra-O-acetyl-beta-D-galactopyranosyl)- (1-->3)-4,6-di-O-acetyl-2-deoxy-2-dimethylmaleimido-alpha,beta-D- glucopyranosyl] trichloroacetimidate as glycosyl donor, to afford with 8 as acceptor the corresponding tetrasaccharide 22, which is transformed into 15, thus giving an alternative approach to 1.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
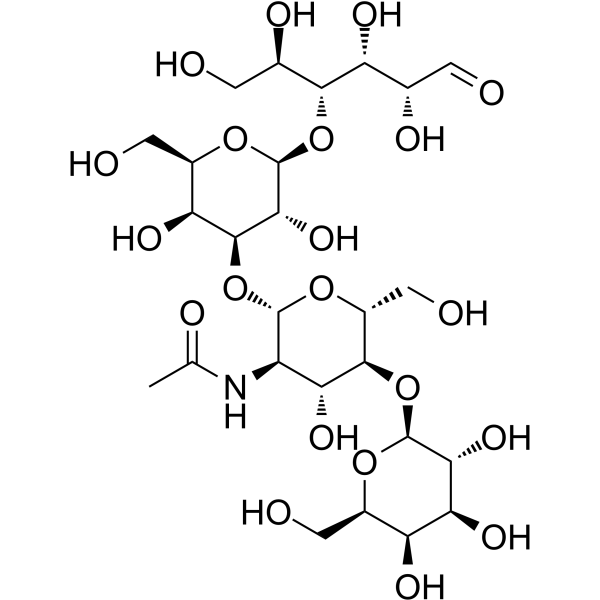 |
乳糖-N-新四糖
CAS:13007-32-4 |
C26H45NO21 |
|
Chemo-enzymatic supported synthesis of the 3-sulfated Lewis ...
2008-04-07 [Carbohydr. Res. 343(5) , 970-6, (2008)] |
|
Enhanced factor H binding to sialylated Gonococci is restric...
2005-11-01 [Infect. Immun. 73(11) , 7390-7, (2005)] |
|
Syntheses of a series of lacto-N-neotetraose clusters using ...
2006-03-20 [Carbohydr. Res. 341(4) , 467-73, (2006)] |
|
In vivo fucosylation of lacto-N-neotetraose and lacto-N-neoh...
2001-06-01 [Glycoconj. J. 18(6) , 465-74, (2001)] |
|
Affinity-purified human immunoglobulin G that binds a lacto-...
2007-02-01 [Infect. Immun. 75 , 1025-1033, (2007)] |