Journal of the American Chemical Society
2008-08-20
Controlled cyclopolymerization through quantitative 19-membered ring formation.
Bungo Ochiai, Yuuko Ootani, Takeshi Endo
文献索引:J. Am. Chem. Soc. 130(33) , 10832-3, (2008)
全文:HTML全文
摘要
Cyclopolymerization of a bis-methacrylate monomer derived from trans-cyclohexanediol and 2-methacryloyloxyethyl isocyanate proceeded through 19-membered ring formation. The unusual large ring formation originated from the designed steric regulation by the cyclohexane ring and the hydrogen bonds. The RAFT polymerization employing cumyl dithiobenzoate attained controlled polymerization, which supported the quantitative cyclization.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
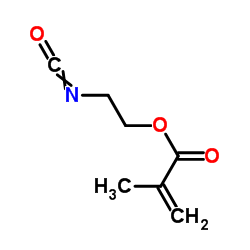 |
甲基丙烯酸异氰基乙酯
CAS:30674-80-7 |
C7H9NO3 |
相关文献:
更多...
|
Construction of monomer-free, highly crosslinked, water-comp...
2014-12-01 [J. Dent. Res. 93(12) , 1326-31, (2014)] |
|
Galectin-3 binding protein links circulating microparticles ...
2015-10-01 [Lupus 24 , 1150-60, (2015)] |
|
Synthesis of antibacterial methacrylate monomer derived from...
2015-09-01 [J. Mech. Behav. Biomed. Mater. 49 , 61-8, (2015)] |
|
Aggregates of mutant CFTR fragments in airway epithelial cel...
2015-03-01 [J. Cyst. Fibros. 14(2) , 182-93, (2015)] |
|
Microcapsules for Enhanced Cargo Retention and Diversity.
2015-06-24 [Small 11 , 2903-9, (2015)] |