Urine from treated cattle drives selection for cephalosporin resistant Escherichia coli in soil.
Murugan Subbiah, Devendra H Shah, Thomas E Besser, Jeffrey L Ullman, Douglas R Call
文献索引:PLoS ONE 7(11) , e48919, (2012)
全文:HTML全文
摘要
The U.S. Food and Drug Administration recently issued new rules for using ceftiofur in food animals in part because of an increasing prevalence of enteric bacteria that are resistant to 3(rd)-generation cephalosporins. Parenteral ceftiofur treatment, however, has limited effects on enteric bacteria so we tested the hypothesis that excreted ceftiofur metabolites exert significant selection pressure for ceftiofur-resistant Escherichia coli in soil. Test matrices were prepared by mixing soil with bovine feces and adding urine containing ceftiofur metabolites (CFM) (0 ppm, ∼50 ppm and ∼100 ppm). Matrices were incubated at 23°C or 4°C for variable periods of time after which residual CFM was quantified using a bioassay. Bla(CMY-2) plasmid-bearing ceftiofur resistant (cef(R)) E. coli and one-month old calves were used to study the selection effects of CFM and transmission of cef(R) bacteria from the environment back to animals. Our studies showed that urinary CFM (∼13 ppm final concentration) is biologically degraded in soil within 2.7 days at 23°C, but persists up to 23.3 days at 4°C. Even short-term persistence in soil provides a >1 log(10) advantage to resistant E. coli populations, resulting in significantly prolonged persistence of these bacteria in the soil (∼two months). We further show that resistant strains readily colonize calves by contact with contaminated bedding and without antibiotic selection pressure. Ceftiofur metabolites in urine amplify resistant E. coli populations and, if applicable to field conditions, this effect is far more compelling than reported selection in vivo after parenteral administration of ceftiofur. Because ceftiofur degradation is temperature dependent, these compounds may accumulate during colder months and this could further enhance selection as seasonal temperatures increase. If cost-effective engineered solutions can be developed to limit ex vivo selection, this may limit proliferation for ceftiofur resistant enteric bacteria while preserving the ability to use this important antibiotic in food animal production.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
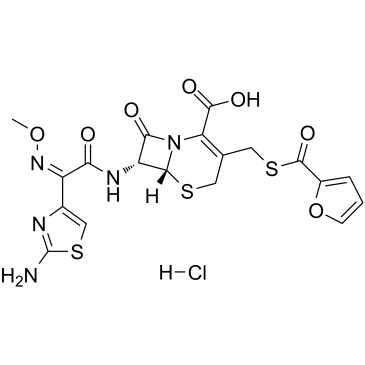 |
盐酸头孢噻呋
CAS:103980-44-5 |
C19H18ClN5O7S3 |
|
blaCTX-M-32 on an IncN plasmid in Escherichia coli from beef...
2013-02-01 [Antimicrob. Agents Chemother. 57(2) , 1096-7, (2013)] |
|
Genetic merit for fertility traits in Holstein cows: V. Fact...
2014-09-01 [J. Dairy Sci. 97(9) , 5543-57, (2014)] |
|
Lipase is essential for the study of in vitro release kineti...
2012-06-04 [Mol. Pharm. 9(6) , 1803-11, (2012)] |
|
Validation study of the BetaStar plus lateral flow assay for...
2012-01-01 [J. AOAC Int. 95(4) , 1211-21, (2012)] |
|
Comparison of enrofloxacin and ceftiofur sodium for the trea...
2012-01-01 [Can. Vet. J. 53(1) , 57-62, (2012)] |