Mapping the substrate-binding site of a human class mu glutathione transferase using nuclear magnetic resonance spectroscopy.
C J Penington, G S Rule
文献索引:Biochemistry 31(11) , 2912-20, (1992)
全文:HTML全文
摘要
The substrate-binding site of a human muscle class mu glutathione transferase has been characterized using high-resolution nuclear magnetic resonance spectroscopy. Isotopic labeling has been used to simplify one-dimensional proton NMR spectra of the Tyr and His residues in the enzyme and two-dimensional carbon-proton spectra of the Ala and Met residues in the enzyme. The resonance lines from 8 of the 12 Tyr residues have been assigned using site-directed mutagenesis. Replacement of Tyr7 with Phe reduced the activity of the enzyme 100-fold. The proximity of His, Tyr, Ala, and Met residues to the active site has been determined using a nitroxide-labeled substrate analogue. This substrate analogue binds with high affinity (Keq = 10(6) M-1) to the enzyme and is a competitive inhibitor. None of the His residues are within 17 A of the active site. Three of the assigned Tyr residues are greater than 17 A from the active site. Quantitative measurement of paramagnetic line broadening of five additional Tyr residues places them within 13-17 A from the active site. Broadening of the Ala and Met resonance lines by the spin-labeled substrate indicates that three Ala residues are 9-16 A from the nitroxide, three Met residues are less than 9 A from the nitroxide, and two Met residues are 9-16 A from the nitroxide.(ABSTRACT TRUNCATED AT 250 WORDS)
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
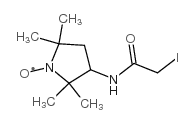 |
3-(2-碘乙酰氨基)-2,2,5,5-四甲基-1-吡咯烷基氧自由基
CAS:27048-01-7 |
C10H18IN2O2 |
|
Characterization of ERM transactivation domain binding to th...
2015-08-18 [Nucleic Acids Res. 43 , 7110-21, (2015)] |
|
Functional role of the flexible N-terminal extension of FKBP...
2013-01-01 [Sci. Rep. 3 , 2985, (2013)] |
|
Escherichia coli antitoxin MazE as transcription factor: ins...
2015-01-01 [Nucleic Acids Res. 43(2) , 1241-56, (2015)] |
|
A fully enzymatic method for site-directed spin labeling of ...
2014-09-01 [Nucleic Acids Res. 42(15) , e117, (2014)] |
|
Role of the C-terminal domain in the structure and function ...
2013-01-01 [Nat. Commun. 4 , 2465, (2013)] |