Syntheses of 2-keto-3-deoxy-D-xylonate and 2-keto-3-deoxy-L-arabinonate as stereochemical probes for demonstrating the metabolic promiscuity of Sulfolobus solfataricus towards D-xylose and L-arabinose.
Robert M Archer, Sylvain F Royer, William Mahy, Caroline L Winn, Michael J Danson, Steven D Bull
文献索引:Chemistry 19(8) , 2895-902, (2013)
全文:HTML全文
摘要
Practical syntheses of 2-keto-3-deoxy-D-xylonate (D-KDX) and 2-keto-3-deoxy-L-arabinonate (L-KDA) that rely on reaction of the anion of ethyl 2-[(tert-butyldimethylsilyl)oxy]-2-(dimethoxy phosphoryl) acetate with enantiopure glyceraldehyde acetonide, followed by global deprotection of the resultant O-silyl-enol esters, have been developed. This has enabled us to confirm that a 2-keto-3-deoxy-D-gluconate aldolase from the archaeon Sulfolobus solfataricus demonstrates good activity for catalysis of the retro-aldol cleavage of both these enantiomers to afford pyruvate and glycolaldehyde. The stereochemical promiscuity of this aldolase towards these enantiomeric aldol substrates confirms that this organism employs a metabolically promiscuous pathway to catabolise the C5-sugars D-xylose and L-arabinose.Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
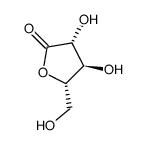 |
L(-)-ARABONIC ACID-GAMMA-LACTONE
CAS:51532-86-6 |
C5H8O5 |
|
Neutral and acidic products derived from hydroxyl radical-in...
2014-04-01 [J. Mass Spectrom. 49 , 280-290, (2014)] |
|
Synthesis of L-hexoses and their related biomolecules.
2013-04-25 [Chem. Commun. (Camb.) 49(32) , 3275-87, (2013)] |
|
The 'true' L-xylulose reductase of filamentous fungi identif...
2010-08-20 [FEBS Lett. 584(16) , 3540-4, (2010)] |
|
Discovery of an L-fucono-1,5-lactonase from cog3618 of the a...
2013-01-08 [Biochemistry 52(1) , 239-53, (2013)] |
|
Extraction of Hemicellulose from Loblolly Pine Woodchips and...
[Ind. Eng. Chem. Res. 52 , 1743-1749, (2013)] |