Mechanism of differential efficacy of garlic organosulfides in preventing benzo(a)pyrene-induced cancer in mice.
S K Srivastava, X Hu, H Xia, H A Zaren, M L Chatterjee, R Agarwal, S V Singh
文献索引:Cancer Lett. 118(1) , 61-7, (1997)
全文:HTML全文
摘要
The mechanism of differential efficacies of diallyl sulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide (DATS), dipropyl sulfide (DPS) and dipropyl disulfide (DPDS) in preventing benzo(a)pyrene (BP)-induced cancer in mice has been investigated by determining their effects on the enzymes of BP activation/inactivation pathways. With the exception of DATS, treatment of mice with other organosulfides (OSCs) caused a small but significant increase (37-44%) in hepatic ethoxyresorufin O-deethylase (EROD) activity. However, the forestomach EROD activity did not differ significantly between control and treated groups. Only DAS treatment caused a modest but statistically significant reduction (about 25%) in pulmonary EROD activity. These results suggest that while reduction of EROD activity may, at least in part, contribute to the DAS-mediated inhibition of BP-induced lung cancer, anticarcinogenic effects of OSCs against BP-induced forestomach carcinogenesis seems to be independent of this mechanism. Treatment of mice with DAS, DADS and DATS resulted in a significant increase, as compared with control, in both hepatic (3.0-, 3.2- and 4.4-fold, respectively) and forestomach (1.5-, 2.7- and 2.7-fold, respectively) glutathione transferase (GST) activity toward anti-7beta,8alpha-dihydroxy-9alpha,10alpha-oxy-7,8,9,10-tetrahydrobenzo(a)pyrene (anti-BPDE), which is the ultimate carcinogen of BP. The pulmonary GST activity was not increased by any of the OSCs. Even though epoxide hydrolase (EH) activity was differentially altered by these OSCs, a correlation between chemopreventive efficacy of OSCs and their effects on EH activity was not apparent. The results of the present study suggest that differences in the ability of OSCs to modulate GST activity toward anti-BPDE may, at least in part, account for their differential chemopreventive efficacy against BP-induced cancer in mice.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
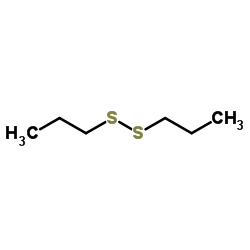 |
二丙基二硫
CAS:629-19-6 |
C6H14S2 | |
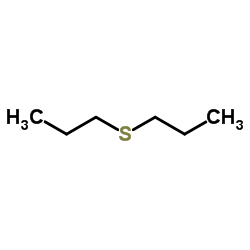 |
二丙基硫醚
CAS:111-47-7 |
C6H14S |
|
Optimizations of packed sorbent and inlet temperature for la...
2014-08-22 [J. Chromatogr. A. 1356 , 221-9, (2014)] |
|
Effect of processing conditions on the organosulfides of sha...
2014-06-11 [J. Agric. Food Chem. 62(23) , 5296-304, (2014)] |
|
Antiapoptotic effects of dietary antioxidants towards N-nitr...
2009-07-01 [J. Appl. Toxicol. 29(5) , 403-13, (2009)] |
|
Organosulfur compounds alone or in combination with vitamin ...
2008-05-09 [Chem. Biol. Interact. 173(1) , 9-18, (2008)] |
|
A preliminary study on the action of genus Allium on thyroid...
1992-03-01 [Rev. Esp. Fisiol. 48(1) , 59-60, (1992)] |