Characteristics of binding of a potent chemotactic formyl tetrapeptide, formylmethionyl-leucyl-phenylalanyl-phenylalanine, to the receptors on rabbit neutrophils.
J C Kermode, N Muthukumaraswamy, R J Freer
文献索引:J. Leukoc. Biol. 43(5) , 420-8, (1988)
全文:HTML全文
摘要
Binding of a potent chemotactic formyl tetrapeptide, formylmethionyl-leucyl-phenylalanyl-phenylalanine (fMet-Leu-Phe-Phe), to the formyl peptide receptors on the rabbit neutrophil was assessed by two approaches. A tritiated preparation of fMet-Leu-Phe-Phe was used for direct binding studies, whereas indirect studies comprised an assessment of the ability of the formyl tetrapeptide to competitively inhibit the binding of 35S-labeled formylmethionyl-leucyl-phenylalanine. These two approaches yielded analogous results. The formyl tetrapeptide fMet-Leu-Phe-Phe showed rapid and saturable binding to the same chemotactic receptors as the less potent formyl tripeptides with which it was compared. Its equilibrium-binding pattern, however, was different: fMet-Leu-Phe-Phe showed a homogeneous binding pattern, in contrast to the heterogeneity seen with the less potent compounds. The relative potencies for high-affinity binding of the two standard formyl tripeptides and fMet-Leu-Phe-Phe correlated well with their relative potencies for stimulating the biological response of degranulation; the relative potencies for low-affinity binding correlated less well.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
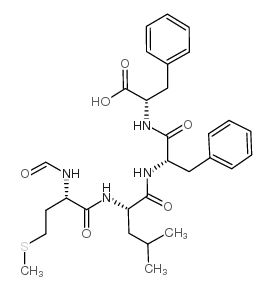 |
N-甲酰-间-亮氨酸-丙氨酸-丙氨酸
CAS:80180-63-8 |
C30H40N4O6S |
|
Formyl peptide chemoattractants: a model of the receptor on ...
1982-01-19 [Biochemistry 21 , 257, (1982)] |
|
Effect of human C-reactive protein on chemokine and chemotac...
1998-09-01 [J. Immunol. 161(5) , 2533-40, (1998)] |
|
Control of fluid shear response in circulating leukocytes by...
2002-03-01 [Ann. Biomed. Eng. 30(3) , 333-43, (2002)] |
|
Interaction of Bordetella pertussis virulence components wit...
1988-08-01 [J. Gen. Microbiol. 134(8) , 2201-11, (1988)] |
|
A novel fluorescent cross-reactive formylpeptide receptor/fo...
2009-03-01 [Cytometry. A 75(3) , 264-70, (2009)] |