Threonine 246 at the active site of the L-lactate dehydrogenase of Bacillus stearothermophilus is important for catalysis but not for substrate binding.
R Sakowicz, H K Kallwass, W Parris, C M Kay, J B Jones, M Gold
文献索引:Biochemistry 32(47) , 12730-5, (1993)
全文:HTML全文
摘要
Threonine 246 is an active site residue that is conserved in all known L-lactate dehydrogenase (LDH; EC 1.1.1.27) sequences. In order to investigate the role of Thr246 in Bacillus stearothermophilus LDH, this residue was altered by site-directed mutagenesis to valine, alanine, leucine, and serine, respectively. The effects of these mutations, as observed in both steady-state and single-turnover kinetic measurements with different substrates, demonstrated the importance for catalysis of a hydroxyl group in the 246 amino acid residue. In contrast, no significant contribution of the OH group of Thr246 to productive pyruvate binding was observed. Instead, it is proposed that the role of Thr246 may be to facilitate hydride transfer from the nicotinamide ring of the NADH cofactor to the pyruvate carbonyl group.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
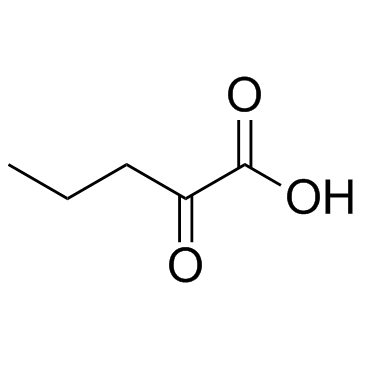 |
2-氧代戊酸
CAS:1821-02-9 |
C5H8O3 |
|
Mosquito odorant receptor for DEET and methyl jasmonate.
2014-11-18 [Proc. Natl. Acad. Sci. U. S. A. 111(46) , 16592-7, (2014)] |
|
An RNA-seq screen of the Drosophila antenna identifies a tra...
2014-11-01 [PLoS Genet. 10(11) , e1004810, (2014)] |
|
Irreversible inhibition of dihydrodipicolinate synthase by 4...
2008-12-01 [Bioorg. Med. Chem. 16 , 9975-83, (2008)] |
|
Chiral hydroxylation at the mononuclear nonheme Fe(II) cente...
2013-01-01 [PLoS ONE 8 , e68932, (2013)] |
|
Cloning and overexpression of ketopantoic acid reductase gen...
2012-02-01 [Appl. Microbiol. Biotechnol. 93(4) , 1619-25, (2012)] |