Spectroscopic analyses on interaction of o-Vanillin-D-Phenylalanine, o-Vanillin-L-Tyrosine and o-Vanillin-L-Levodopa Schiff Bases with bovine serum albumin (BSA).
Jingqun Gao, Yuwei Guo, Jun Wang, Zhiqiu Wang, Xudong Jin, Chunping Cheng, Ying Li, Kai Li
文献索引:Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 78(4) , 1278-86, (2011)
全文:HTML全文
摘要
In this work, three o-Vanillin Schiff Bases (o-VSB: o-Vanillin-D-Phenylalanine (o-VDP), o-Vanillin-L-Tyrosine (o-VLT) and o-Vanillin-L-Levodopa (o-VLL)) with alanine constituent were synthesized by direct reflux method in ethanol solution, and then were used to study the interaction to bovine serum albumin (BSA) molecules by fluorescence spectroscopy. Based on the fluorescence quenching calculation, the bimolecular quenching constant (K(q)), apparent quenching constant (K(sv)), effective binding constant (K(A)) and corresponding dissociation constant (K(D)) as well as binding site number (n) were obtained. In addition, the binding distance (r) was also calculated according to Foster's non-radioactive energy transfer theory. The results show that these three o-VSB can efficiently bind to BSA molecules, but the binding array order is o-VDP-BSA>o-VLT-BSA>o-VLL-BSA. Synchronous fluorescence spectroscopy indicates that the o-VDP is more accessibility to tryptophan (Trp) residues of BSA molecules than to tyrosine (Tyr) residues. Nevertheless, the o-VLT and o-VLL are more accessibility to Tyr residues than to Trp residues.Copyright © 2010 Elsevier B.V. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
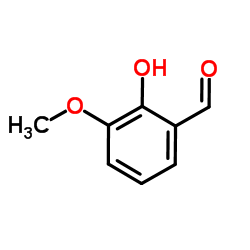 |
邻香草醛
CAS:148-53-8 |
C8H8O3 |
|
3D-QSAR and molecular docking studies of benzaldehyde thiose...
2007-03-01 [Bioorg. Med. Chem. 15 , 2006-15, (2007)] |
|
A chemical screening approach reveals that indole fluorescen...
2009-09-01 [Bioorg. Med. Chem. Lett. 19 , 4952-7, (2009)] |
|
Design, synthesis and characterization of novel binary V(V)-...
2015-06-01 [J. Inorg. Biochem. 147 , 99-115, (2015)] |
|
Cu(II)-Dy(III) and Co(III)-Dy(III) based single molecule mag...
2015-08-07 [Dalton Trans. 44 , 13242-9, (2015)] |
|
Single-Molecule Magnetism, Enhanced Magnetocaloric Effect, a...
2015-10-26 [Chemistry 21 , 15639-50, (2015)] |