Identification of protonation state by XPS, solid-state NMR, and DFT: characterization of the nature of a new theophylline complex by experimental and computational methods.
Joanna S Stevens, Stephen J Byard, Christopher A Muryn, Sven L M Schroeder
文献索引:J. Phys. Chem. B 114(44) , 13961-9, (2010)
全文:HTML全文
摘要
Recent studies suggested that X-ray photoelectron spectroscopy (XPS) sensitively determines the protonation state of nitrogen functional groups in the solid state, providing a means for distinguishing between co-crystals and salts of organic compounds. Here we describe how a new theophylline complex with 5-sulfosalicylic acid dihydrate was established as a salt by XPS prior to assignment with conventional methods. The presence of a C=NH(+) (N9) N1s peak in XPS allows assignment as a salt, while this peak is clearly absent for a theophylline co-crystal. The large low frequency shift for N9 observed by (15)N solid-state nuclear magnetic resonance spectroscopy (ssNMR) and corresponding density functional theory (DFT) calculations confirm that protonation has occurred. The crystal structure and further analytical studies confirm the conclusions reached with XPS and ssNMR. This study demonstrates XPS as an alternative technique for determining whether proton transfer has occurred in acid-base complexes.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
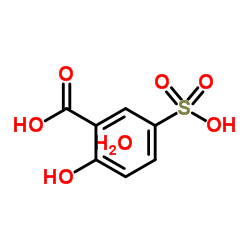 |
5-磺基水杨酸,二水合物
CAS:5965-83-3 |
C7H10O8S |
|
Brief reports: Lysosomal cross-correction by hematopoietic s...
2015-01-01 [Stem Cells 33(1) , 301-9, (2014)] |
|
Self-immolative polycations as gene delivery vectors and pro...
2015-02-02 [Mol. Pharm. 12(2) , 332-41, (2015)] |
|
In vitro evaluation of inorganic mercury and methylmercury e...
2014-12-01 [Food Chem. Toxicol. 74 , 349-59, (2015)] |
|
Rapid determination of PEGylated liposomal doxorubicin and i...
2002-11-05 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 779(2) , 259-69, (2002)] |
|
Spray reagent for the detection of amino acids on thin layer...
1993-02-01 [Amino Acids 4 , 193, (1993)] |