Lipase-catalysed enantioselective ammonolysis of phenylglycine methyl ester in organic solvent.
Wei Du, Minhua Zong, Yong Guo, Dehua Liu
文献索引:Biotechnol. Appl. Biochem. 38(Pt 2) , 107-10, (2003)
全文:HTML全文
摘要
Ammonium, provided by ammonium carbamate as a novel acyl acceptor, was adopted for enzymic enantioselective ammonolysis of racemic phenylglycine methyl ester in this paper and it has been found that the reaction conditions have profound effects on enzymic activity and enantioselectivity: the optimal concentration of ammonium carbamate was 80 mM; t-butanol was the most suitable reaction medium and the optimum initial water activity was found to be 0.75; relatively high reaction rate and enantioselectivity could be attained within the temperature range of 30-40 degrees C. Compared with the corresponding hydrolysis and alcoholysis, enzymic ammonolysis expressed rather high enzymic activity and enantioselectivity and 95% of enzymic activity remained after 10 batches of ammonolysis, which demonstrates that lipase-catalysed ammonolysis is a promising method for the preparation of optically pure (R)-phenylglycine and its derivatives.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
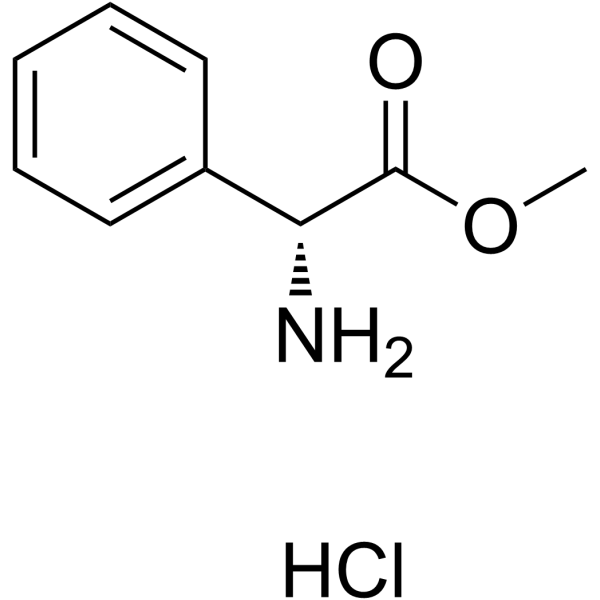 |
D-苯甘氨酸甲酯盐酸盐
CAS:19883-41-1 |
C9H12ClNO2 |
|
Improving lipase-catalyzed enantioselective ammonolysis of p...
2003-03-01 [Biotechnol. Lett. 25(6) , 461-4, (2003)] |
|
Kinetics of ampicillin synthesis catalyzed by penicillin acy...
2000-12-01 [Biochemistry. (Mosc.) 65(12) , 1367-75, (2000)] |
|
Enhanced enzymatic synthesis of a semi-synthetic cephalospri...
2003-07-01 [Biotechnol. Lett. 25(14) , 1195-8, (2003)] |
|
Life cycle assessment of a coupled solar photocatalytic-biol...
2006-11-01 [Water Res. 40(19) , 3533-40, (2006)] |
|
Increasing the synthetic performance of penicillin acylase P...
2004-07-01 [Protein Eng. Des. Sel. 17(7) , 571-9, (2004)] |