Organic Letters
2007-01-18
Synthesis of protected chiral vicinal diaminoalcohols by diastereoselective intramolecular benzylic substitution from bistrichloroacetimidates.
Christophe Rondot, Pascal Retailleau, Jieping Zhu
文献索引:Org. Lett. 9 , 247, (2007)
全文:HTML全文
摘要
An efficient synthesis of chiral dihydrooxazines (2) from 1-aryl-2-amino-propane-1,3-diols (1) via the corresponding bistrichloroacetimidate intermediates has been developed. In this transformation, one trichloroacetimidate acts as a leaving group and the other acts as a nucleophile. The cyclization proceeds through an SN1 mechanism to provide trans-dihydrooxazines with complete diastereoselectivity irrespective of the absolute configuration of the benzylic alcohol. The transformation of 2 into other selectively protected aminodiols is also documented. [reaction: see text].
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
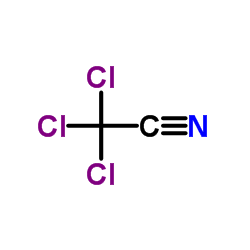 |
三氯乙腈
CAS:545-06-2 |
C2Cl3N |
相关文献:
更多...
|
Identification of two urinary metabolites of rats treated wi...
1981-02-01 [Toxicol. Lett. 7(4-5) , 321-7, (1981)] |
|
Trichloroacetonitrile.
1999-01-01 [IARC Monogr. Eval. Carcinog. Risks Hum. 71 Pt 3 , 1533-7, (1999)] |
|
Teratogenic effects of trichloroacetonitrile in the Long-Eva...
1988-08-01 [Teratology 38(2) , 113-20, (1988)] |
|
Genotoxic activity of five haloacetonitriles: comparative in...
2000-01-01 [Environ. Mol. Mutagen. 36(1) , 52-8, (2000)] |
|
Characterization of dissolved organic matter fractions and i...
2009-01-01 [J. Environ. Sci. (China) 21(1) , 54-61, (2009)] |