A quantitative study of the biospecific desorption of rat liver (M4) lactate dehydrogenase from 10-carboxydecylamino-Sepharose. Determination of the number of ligand-binding sites blocked on adsorption.
P Kyprianou, R J Yon
文献索引:Biochem. J. 207(3) , 549-56, (1982)
全文:HTML全文
摘要
1. The theory of Nichol, Ogston, Winzor & Sawyer [(1974) Biochem. J. 143, 435-443] for quantitative affinity chromatography, when adapted for use with a non-specific column from which a multi-site protein can be specifically desorbed by its free ligand, permits determination of the concentration of adsorption sites on the column, their adsorptive affinity (as an association constant) and either the intrinsic (site) constant for ligand-binding to the protein or an 'occlusion coefficient' (defined as the number of ligand-binding sites blocked on adsorption), one of which must be known. 2. The theory has been applied to the NADH-specific desorption of rat liver M4 lactate dehydrogenase from 10-carboxydecylamino-Sepharose. It suggests that most of the enzyme molecules are adsorbed with at least two NADH-binding sites blocked, indicating an extensive adsorption interface in relation to the protein surface. Other chromatographic parameters were also determined for the system. 3. Among topics discussed are (a) factors affecting the experimentally determined value for the number of blocked sites, (b) the nature of the adsorption sites on the column and (c) the similarity of the analysis to that for determining Hill coefficients, and other possible applications.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
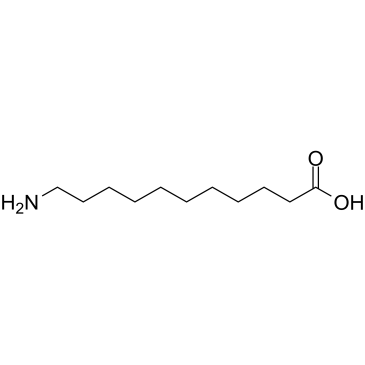 |
11-氨基十一酸
CAS:2432-99-7 |
C11H23NO2 |
|
Advanced material and approach for metal ions removal from a...
2015-01-01 [Sci. Rep. 5 , 8992, (2015)] |
|
Development of 68Ga-labeled fatty acids for their potential ...
2014-06-15 [J. Labelled Comp. Radiopharm. 57(7) , 463-9, (2014)] |
|
Biocompatibility of Fe(3)O(4) nanoparticles evaluated by in ...
2010-02-19 [Nanotechnology 21 , 75102, (2010)] |
|
11-Aminoundecanoic acid.
1986-01-01 [IARC Monogr. Eval. Carcinog. Risk Chem. Hum. 39 , 239-45, (1986)] |
|
Tailoring aqueous solubility of functionalized single-wall c...
2005-10-01 [Nano Lett. 5(10) , 2001-4, (2005)] |