Synthesis of lactones using substituted benzoic anhydride as a coupling reagent.
Isamu Shiina, Hiroki Fukui, Akane Sasaki
文献索引:Nat. Protoc. 2(10) , 2312-7, (2007)
全文:HTML全文
摘要
This protocol describes a procedure for the synthesis of a 29-membered macrolactone. The facile mixed-anhydride method is very effective for the preparation of carboxylic esters and lactones using substituted benzoic anhydrides by the promotion of Lewis acid or basic catalysts under mild reaction conditions. Owing to the reaction rapidly proceeding to produce the monomeric compounds with high chemoselectivity, the protocol is quite powerful for the synthesis of not only the giant-sized lactones but also the highly strained cyclic compounds such as medium-sized lactones. The remarkable efficiency of the lactonizations promoted by the substituted benzoic anhydrides has been already shown in the synthesis of many natural complex molecules. It takes approximately 19 h to complete the protocol: 0.5 h to set up the reaction, 13.5 h for the reaction and 5 h for isolation and purification.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
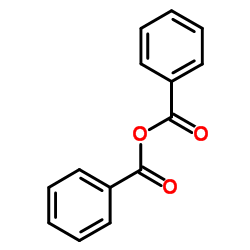 |
苯甲酸酐
CAS:93-97-0 |
C14H10O3 |
|
Regioselective benzoylation of glycopyranosides by benzoic a...
2012-10-01 [Carbohydr. Res. 359 , 111-9, (2012)] |
|
Introduction of a benzoyl group onto 6-chloropurine riboside...
2000-01-01 [Nucleic Acids Symp. Ser. 44 , 103-104, (2000)] |
|
Derivatization for LC-electrospray ionization-MS: a tool for...
2004-05-15 [Anal. Chem. 76(10) , 2869-77, (2004)] |
|
An effective use of benzoic anhydride and its derivatives fo...
2004-03-19 [J. Org. Chem. 69(6) , 1822-30, (2004)] |
|
Ru(II) multinuclear metallosupramolecular rack-type architec...
2010-05-17 [Chemistry 16(19) , 5645-60, (2010)] |