Protonation and silver(I) complex-formation equilibria of some amino-alcohols.
S Canepari, V Carunchio, P Castellano, A Messina
文献索引:Talanta 44 , 2059-67, (1997)
全文:HTML全文
摘要
Formation constants of the silver(I) complexes with some amino-alcohols have been determined at 25 degrees C in 0.5 M KNO(3) by means of two independent potentiometric measurements employing glass and silver electrode. The ligands considered are: sec-butylamine, 2-amino-1-propanol, 2-amino-1-methoxy-propane, 2-amino-2-methyl-1-propanol, 2-amino-1-butanol, 2-amino-1-pentanol, 2-amino-1-hexanol, 2-amino-1,3-propanediol, 2-amino-1,3-hexandiol, 2-amino-2-methyl-1,3-propanediol and Tris(hydroxymethyl)-aminomethane. Protonation constants of the selected ligands have also been determined. Calculations were made using HYPERQUAD computer program. The influence exerted by the introduction of hydroxy groups and by the presence of alkyl residuals in the ligand structure on the formation equilibria, is discussed.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
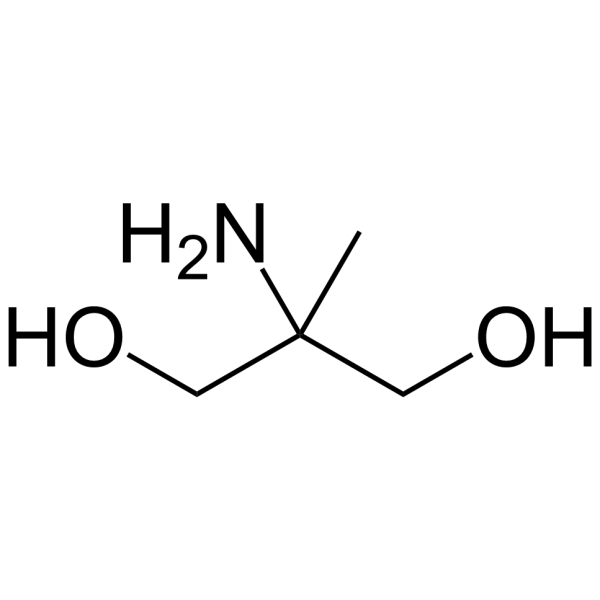 |
2-氨基-2-甲基-1,3-丙二醇(AMPD)
CAS:115-69-5 |
C4H11NO2 |
|
Immunophenotype and gene expression profile of mesenchymal s...
2014-09-15 [Vet. Immunol. Immunopathol. 161(1-2) , 21-31, (2014)] |
|
Gliotoxin potentiates osteoblast differentiation by inhibiti...
2015-07-01 [Mol. Med. Report. 12 , 877-84, (2015)] |
|
Final amended report on safety assessment on aminomethyl pro...
2009-01-01 [Int. J. Toxicol. 28(6 Suppl) , 141S-61S, (2009)] |
|
Biomineral/Agarose Composite Gels Enhance Proliferation of M...
2015-01-01 [Int. J. Mol. Sci. 16 , 14245-58, (2015)] |
|
Generalized polymer effective charge measurement by capillar...
2014-11-28 [J. Chromatogr. A. 1370 , 255-62, (2014)] |