Glycyl glutamine, an inhibitory neuropeptide derived from beta-endorphin.
D C Parish, D G Smyth, J R Normanton, J H Wolstencroft
文献索引:Nature 306 , 267, (1983)
全文:HTML全文
摘要
The primary mechanism of activation of intracellular prohormones seems to involve proteolytic cleavage at sequences of consecutive basic residues. Thus, all the known biologically active peptides derived from the prohormone of corticotropin and beta-endorphin appear to be excised initially by enzymes with this specificity. The C-terminal peptide, beta-endorphin (1-31), is generated by cleavage at a lysyl arginine sequence and an additional cleavage can give rise to the related peptides, beta-endorphin (1-27) and beta-endorphin (1-26). These derivatives of beta-endorphin are released by an endopeptidase that appears to catalyse cleavage on the carboxyl side of paired lysine residues, followed by the action of a carboxypeptidase B-like enzyme (Fig. 1). The beta-endorphin fragments, beta-endorphin (1-27) and beta-endorphin (1-26), have been isolated from porcine and bovine pituitary but the C-terminal dipeptide, glycyl glutamine, has not been reported previously. Here we describe the isolation of glycyl glutamine from porcine pituitary and present evidence for its presence in sheep brain stem. When applied ionophoretically to brain stem neurones in the rat, the dipeptide exhibited an inhibitory action on cell firing.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
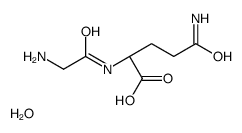 |
甘氨酰-L-谷氨酰胺
CAS:172669-64-6 |
C7H15N3O5 |
|
Influence of two glutamine-containing dipeptides on growth o...
1988-07-01 [In Vitro Cell. Dev. Biol. 24 , 696, (1988)] |
|
Glutamine-based dipeptides are utilized in mammalian cell cu...
1994-11-15 [J. Biotechnol. 37(3) , 277-90, (1994)] |
|
Ammonium accumulation in commercially available embryo cultu...
2016-06-01 [Hum. Reprod. 31(6) , 1192-9, (2016)] |
|
Glycyl-L-glutamine, a precursor, and glycyl-L-glutamic acid,...
1985-08-01 [Proc. Natl. Acad. Sci. U. S. A. 82 , 5213, (1985)] |
|
Parish, D.C. and Smyth, D.G.
[Biochem. Soc. Trans. 10 , 221, (1982)] |