Comparative analysis of activator-Esigma54 complexes formed with nucleotide-metal fluoride analogues.
Patricia C Burrows, Nicolas Joly, B Tracy Nixon, Martin Buck
文献索引:Nucleic Acids Res. 37(15) , 5138-50, (2009)
全文:HTML全文
摘要
Bacterial RNA polymerase (RNAP) containing the major variant sigma(54) factor forms open promoter complexes in a reaction in which specialized activator proteins hydrolyse ATP. Here we probe binding interactions between sigma(54)-RNAP (Esigma(54)) and the ATPases associated with various cellular activities (AAA+) domain of the Escherichia coli activator protein, PspF, using nucleotide-metal fluoride (BeF and AlF) analogues representing ground and transition states of ATP, which allow complexes (that are otherwise too transient with ATP) to be captured. We show that the organization and functionality of the ADP-BeF- and ADP-AlF-dependent complexes greatly overlap. Our data support an activation pathway in which the initial ATP-dependent binding of the activator to the Esigma(54) closed complex results in the re-organization of Esigma(54) with respect to the transcription start-site. However, the nucleotide-dependent binding interactions between the activator and the Esigma(54) closed complex are in themselves insufficient for forming open promoter complexes when linear double-stranded DNA is present in the initial closed complex.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
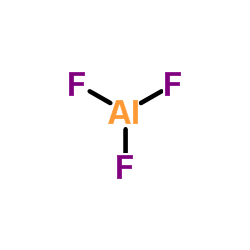 |
氟化铝
CAS:7784-18-1 |
AlF3 |
|
Comparison of the Biological Impacts of the Fluoride Compoun...
2015-09-01 [Biol. Trace Elem. Res. 167 , 84-90, (2015)] |
|
Protective effect of Spirulina and tamarind fruit pulp diet ...
2012-12-01 [Indian J. Exp. Biol. 50(12) , 897-903, (2012)] |
|
An in vitro study of the effect of aluminum and the combined...
2012-06-01 [Biol. Trace Elem. Res. 147(1-3) , 418-27, (2012)] |
|
Preparation and stabilization of aluminium trifluoroacetate ...
2012-10-07 [Dalton Trans. 41(37) , 11351-60, (2012)] |
|
Cooling-increased phospho-β-arrestin-1 and β-arrestin-1 expr...
2012-08-01 [Cryobiology 65(1) , 12-20, (2012)] |