| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
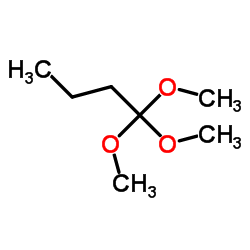 |
原丁酸三甲酯
CAS:43083-12-1 |
|
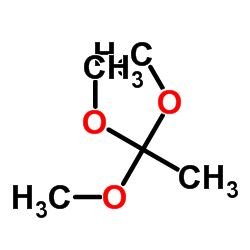 |
原乙酸三甲酯
CAS:1445-45-0 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
原丁酸三甲酯
CAS:43083-12-1 |
|
 |
原乙酸三甲酯
CAS:1445-45-0 |